 Mini Review
Mini Review
State-Of-The-Art Ultrasound in the Management of the Infertile Couple
Tara Giacchino*, Rebecca Karkia and Hasib Ahmed
Institute of Medical Sciences, Canterbury Christ Church University, United Kingdom
Tara Giacchino, Institute of Medical Sciences, Canterbury Christ Church University, United Kingdom.
Received Date: October 19, 2020; Published Date: November 10, 2020
Abstract
Ultrasound is vital tool for imaging women and men with infertility as it is inexpensive, accessible and non-invasive, providing crucial information to allow quick diagnosis. It also helps to facilitate an interactive discussion with the patient where findings can instantly be seen and acted upon. Additionally, US is indispensable for assisting IVF and ICSI treatment especially with the introduction of 3D technology ensuring the best quality oocytes are used to strive for the best outcomes. The diagnosis and treatment of infertility can be extremely distressing for patients and we must ensure we provide the highest level of care with the use of US playing a key role in management. Furthermore, all contemporary trainees aspiring for a career in reproductive medicine should be trained and competent in advanced ultrasound.
Keywords: Female infertility; Male infertility; Ultrasound; 3D imaging; Ovarian reserve; Assisted reproductive technology; Oocyte quality; Oocyte retrieval; Embryo implantation; Embryo transfer
Introduction
Infertility affects 1 in 7 heterosexual couples in the UK and is diagnosed when a woman of reproductive age has not become pregnant after at least 12 months of timed unprotected intercourse or 6 cycles of donor insemination. Earlier evaluation after 6 months may be required in certain circumstances such as, maternal age over 36 years or known history of predisposing factors to infertility [1]. Ultrasound (US) plays a crucial role in all aspects of the management of the infertile couple with the introduction of three-dimensional (3D) imaging and the use of doppler US greatly improving our knowledge on ovarian function and quality [2]. The purpose of this article is to summarize the crucial role US has in the investigation and diagnosis of the infertile couple as well as assisting in the creation and implantation of an embryo in assisted reproductive techniques.
Discussion
Investigation of the infertile couple
Ovarian Reserve: Ovarian reserve is determined by the number and quality of primordial follicles and is reflective of a woman’s reproductive potential. There is no universal consensus to define decreased ovarian reserve but is usually understood as a reduced response to either ovarian stimulation or regular intercourse in women who are ovulating and trying to conceive [3].
There are no existing tests to estimate the true ovarian reserve, but this can be indirectly estimated using US to measure the ovarian antral follicle count (AFC) [4]. Primordial follicles grow in diameter from less than 0.05mm (impossible to see on US) to 2mm, when they develop an antral cavity lined by granulosa cells, capable of producing estrogen, and are consequently called antral follicles.
AFC is measured using transvaginal US (TVUS) and is reported as the total number of antral follicles measuring 2 to 10mm in diameter from both ovaries [5]. With the recent novel use of 3D US and advancements in image resolution, follicles less than 2mm in diameter can be counted, however there is debate on whether they should be included as such small follicles would not respond well to follicle-stimulating hormone(FSH) [6]. Unfortunately, the technique to count antral follicles is not standardized and can result in differences secondary to the operator and the type of equipment used [7]. Table 1 summarizes the different US modalities to count antral follicles.
Table 1: Different ultrasound modalities for measuring Antral Follicle Count [5].
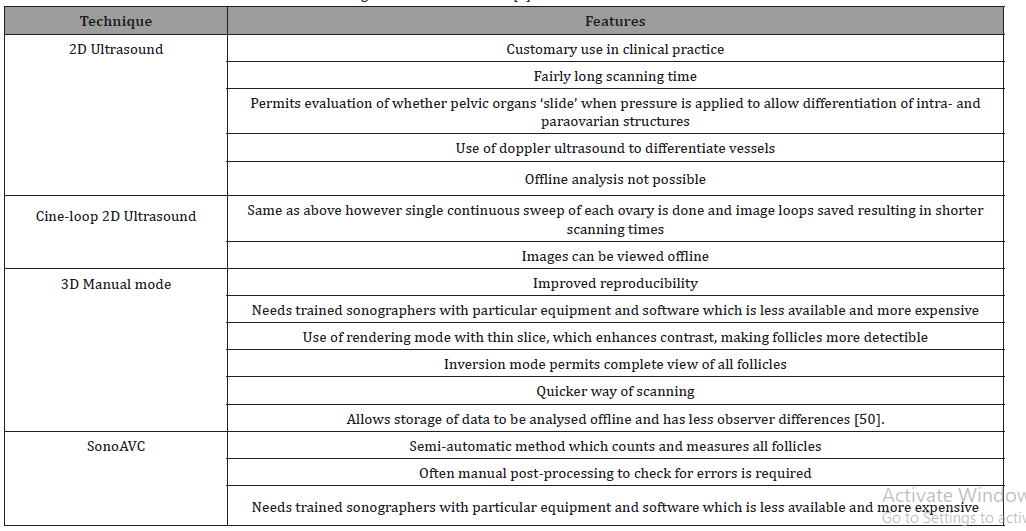
The serum level of Anti Mullerian Hormone (AMH), released by granulosa cells of antral follicles, can also be used to estimate ovarian reserve. Ovarian reserve also helps to evaluate the potential response to ovarian stimulation in assisted reproductive techniques (ART) and so allows tailoring of treatment regimens ensuring best results with minimal risks i.e. avoiding ovarian hyperstimulation syndrome (OHSS). AMH and AFC have comparable accurateness with prediction of ovarian reserve and stimulation response [8] and both can be measured at any time in the menstrual cycle [9,10]. Using US to measure AFC may be technically challenging at times with poor views due to reduced mobility of the ovaries or because of lack of operator experience. AFC also gives no indication of the quality of the embryo [11]. In these circumstances the use of AMH is useful [11] even though, the assays may take hours to give the final value and discrepancies with handling, storage and interpretation of results many exist between different laboratories [12].
The use of US can be thought of as “superior” as it gives more insight into ovarian morphology and reinforces the prediction of ovarian reserve and response to stimulation by measure of ovarian volume and use of doppler. Spectral doppler is used to measure pulsatility index (PI) and peak systolic velocity (PSV) of stromal intraovarian blood flow. Mean stromal PSV of 10cm/s is indicative of a normal response to stimulation or the ovulation trigger, 5cm/s is a low response and 15cm/s indicates a high response to stimulation and so increased risk of OHSS [13,14].
Investigation of the infertile male: Findings from a systematic review in 2015 recommended the use of US as part of the investigation of male infertility as it provides detailed assessment of the male genital tract. US detects abnormal anatomy such as epididymis dilatation (indicative of obstruction) or congenital abnormalities such as absence of vas deferens as well as allowing more accurate measurement of testicular volume compared to the Prader orchidometer when pathology is present e.g. large hydrocele. US can assess testicular blood flow to identify testicular torsion (decreased flow) or epididymo-orchitis (hyperaemia) with the added use of colour doppler to measure venous reflux to identify a varicocoele when palpation isn’t possible. Furthermore, trans-rectal US (TRUS) is used to detect distal anomalies of the vas deferens related to ejaculatory duct obstruction seen in obstructive azoospermia as well as prostatic abnormalities such as a small or large prostate (related to hypogonadism and aging or metabolic abnormalities) or prostate and seminal vesicle ecotecture abnormalities which are linked to inflammation or stasis of the vesicles. Finally, TRUS can assist in testicular sperm extraction [15].
Polycystic Ovarian Syndrome (PCOS): PCOS can manifest in anovulatory cycles and is a chief cause of infertility found in 8-13% of women of reproductive age with as many as 70% of those affected being left undiagnosed [16]. There is a huge amount of debate surrounding the diagnosis and management of PCOS, but the consensus is that the root cause of this disease is ovarian dysfunction. US is routinely used to diagnose ovaries with polycystic morphology (PCOM) however their presence does not confirm PCOS but is one of the diagnostic features [17].
The release of luteinizing hormone (LH) from the anterior pituitary is in surplus compared to FSH resulting in ovarian hyperthecosis, increased ovarian volume and increased androgen production. The ovarian follicles also secrete an increased amount of AMH which causes more angiogenic factors to be released such as vascular endothelial growth factor (VGEF) in the ovarian stroma [18]. AMH makes ovarian follicles less receptive to FSH; this ceases their growth between 2 and 6mm leading to a rapid upsurge of antral follicles [19]. US can detect this increased ovarian volume, stroma and number of antral follicles [20].
The Rotterdam consensus conference defines PCOM as the “presence of 12 or more follicles in at least one ovary measuring 2-9mm in diameter, and/or increased ovarian volume (>10mL)” [21]. PCOM when stimulated behave similarly to ovaries in women with PCOS in view of increased VGEF and in both scenarios there is an increased risk of hyperstimulation and OHSS. The increased presence of angiogenic factors is manifested as high stromal velocities, and if this is detected on US before commencing in vitro fertilization (IVF), the dose of stimulation can be decreased accordingly. Women with PCOM or PCOS need to be monitored closely for changes in stromal velocities or estradiol levels and could possibly be treated prophylactically with buserlin or freezing of embryos [22].
Ovarian cysts: US is used to diagnose ovarian cysts which could potentially cause delay in starting fertility treatment such as cysts secreting estradiol, malignant cysts or those affecting the menstrual cycle [2] In 2015, Hamdan D, et al. [23] conducted a systematic review and meta-analysis including 33 studies and concluded that women with endometriomas undergoing IVF or intracytoplasmic sperm injection (ICSI) have comparable reproductive outcomes to women without theses cysts, so long as deep infiltrating endometriosis wasn’t also present. They did however notice a higher cycle cancellation rate. Furthermore, surgical removal of the endometrioma did not improve reproductive outcomes.
Hysterosalpingo Contrast Sonography (HyCoSy): Laparoscopy is considered the gold standard for diagnosing tubal patency however HyCoSy allows a quick, easy and non-invasive assessment of the uterine cavity and tubes. Contrast injection in given through the cervix thereby giving an image of the uterine cavity and fallopian tubes by means of TVUS [24]. When compared to hysterosalpingography (HSG), HyCoSy has a higher sensitivity (90%) for detecting synechiae, polyps and submucosal myomas [25]. In 2017, Vickramarajah S, et al [26] reported a sensitivity of 96% in picking up tubal blockage.
The use of 3D contrast-enhanced hysterosalpingosonography with gel foam (HyFoSy) has been suggested as a safe alternative instead of the standard microbubbles suspended in contrast fluid with the added advantage that the foam remains stable and allows the entire tube to be assessed. Studies have shown that HyFoSy is quicker, more comfortable for the patient with less infection rates and has increased spontaneous pregnancy rates however is not widely used yet because it requires operator training and FDA approval of the foam [27].
Creation of an Embryo
Follicular growth: At a diameter of 6mm, antral follicles develop FSH receptors which allow cyclical growth driven by FSH [28]. US is the only way follicular growth can be tracked in natural cycles and in IVF or ICSI post stimulation by administered FSH. Fertility specialists detect the mean follicular diameter which correlates best with a “mature” oocyte ready for the ovulation trigger. Table 2 summarizes the differences between 2D and 3D US for follicular tracking.
Table 2: Comparison of 2D and 3D US for follicular tracking.
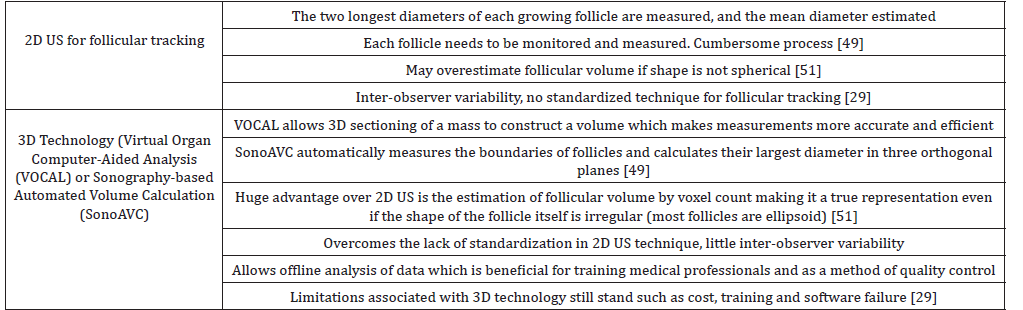
In 2014, Revelli A, et al. [29] published a review which looked at 15 years’ worth of literature to compare the two US techniques and the final IVF outcome. The main conclusion from this study is that 2D US has its limitations as discussed in table 2 however large prospective studies are needed to ratify the superiority of 3D technology.
Oocyte quality: Assessment of oocyte quality is equally as important with evidence showing that ultrasound measurement of perifollicular blood flow by colour and spectral doppler is a good indicator. The perifollicular velocity is a reflection of the oxygen content of the follicle with a PSV of more than 10cm/s at the time of ovulation trigger correlating with optimal fertilization and a good quality embryo. This is especially important in mild or natural IVF cycles where the timing of ovulation trigger is imperative [30,31].
A small prospective study (26 patients) carried out by Robson et al revealed a statistically significant increase in pregnancy rate when the embryo transfer cohort consisted of at least one highly vascular follicle [32].
Oocyte retrieval in IVF: The goal is to collect the greatest number of oocytes from ovarian follicles in the least invasive way. US completely revolutionized oocyte retrieval as this was traditionally done by means of a laparotomy then eventually laparoscopically making it an invasive risky procedure. Oocyte collection is now performed via ultrasound guided transvaginal aspiration. Follicles over 14mm are aspirated using low-pressure suction with colour doppler identifying any blood vessels which need to be avoided to prevent any intraperitoneal bleeding [33].
A randomised control trial compared transvaginal approach to laparoscopy concluded that the number of oocytes collected, the number of embryos recovered, and the pregnancy rates were comparable [34]. The transvaginal route also has the added benefit of being less invasive, more cost effective with a 0.4% complication rate (including intraperitoneal bleeding, infection and anaesthetic risks) making it the favoured method for oocyte retrieval worldwide [35].
Implantation of the embryo
Endometrial factors: The use of US in ART allows assessment of the endometrial vascular changes and angiogenesis in preparation for implantation; abnormal vascularity may lead to implantation failure with subsequent early pregnancy loss [36]. These changes occur during an ‘implantation window” when endometrial pinopods develop at the LH surge at around day 9 in a natural cycle. US can help time the ovulation trigger in IVF or frozen embryo transfer cycles to ensure it occurs during this implantation window by measuring the endometrial thickness (ET) and using power doppler.
Richter and colleagues carried out a retrospective cohort study including 1,294 IVF cycles and showed that the clinical pregnancy rate significantly increased with increasing ET irrespective of the patients age and embryo quality. They also noted a trend towards decreasing spontaneous miscarriage [37]. Zhao and colleagues looked at the ET together with endometrial pattern on the day of ovulation trigger of 1933 IVF patients and confirmed that ET of more than 9mm has a high sensitivity and specificity for predicting pregnancy outcome. The endometrial pattern independently affects pregnancy outcomes with a triple layer endometrium being more favorable [38].
Vascular invasion of the endometrium assessed by TVUS power doppler is measured before the ovulation trigger with studies showing that sonographic detection of good endometrial and sub endometrial flow correlates with improved clinical pregnancy rates [39]. Finally, abnormal uterine artery pulsatility index correlates with lower implantation rates and these women should be advised to freeze their embryos and subsequently treated with subcutaneous oestrogen and aspirin to improve their uterine vascularity [40].
Uterine factors: US detects uterine abnormalities which may lessen the chances of successful implantation in IVF cycles such as submucous and intramural fibroids which respectively, may cause a 70% and 21% reduction in clinical pregnancy rates and a 70% and 15% reduction in delivery rates [41,42]. Fibroids can subsequently be treated to improve pregnancy outcomes [43]. US can also identify Mullerian duct abnormalities found in 7.3% of infertile women, with comparable sensitivity to MRI [44,45]. These uterine anomalies such as septate or bicornuate uterus do not cause infertility per se but are associated with miscarriage and preterm delivery [46] and once detected can be treated. US can also detect adenomyosis which can be one of the factors contributing to infertility, specifically a 40% reduction in clinical pregnancy rate and 40% increase in miscarriage [47], however there is little that can be done to improve fertility in this scenario.
Embryo Transfer
Successful US guided embryo transfer is the crux of IVF treatment. Transabdominal US accurately guides the embryo transfer catheter through the internal cervical to the endometrium thereby allowing direct visualization to minimize disruption of the endometrium. A meta-analysis including 14 randomised control trials showed that better clinical pregnancy rates (8%) were associated with US guided embryo transfer when compared to the clinical touch method for embryo transfer (4%) [48].
Future Recommendations
In 2019, Levaillant JM, et al [27] proposed an innovative onestop “Fertiliscan” which combines advanced 3D technology and HyFoSy. Fertiliscan can detect ovarian, uterine or endometrial abnormalities, estimate ovarian reserve and finally assess tubal patency with use of HyFoSy. As it is one examination it is cheaper and requires only an hour to provide the answers which would traditionally be given over a number of appointments. For the infertile couple, time is everything and this new technology has the potential to fast track the whole process and reduce the time taken to conceive.
Conclusion
US is the perfect tool for imaging women and men with infertility as it is inexpensive, accessible and non-invasive providing crucial information to allow quick diagnosis, facilitating an interactive discussion with the patient where findings can instantly be discussed. The diagnosis and treatment of infertility can be extremely distressing for patients and we must ensure we provide the highest level of care with the use of US playing a key role in management. Furthermore, all contemporary trainees aspiring for a career in reproductive medicine should be trained and competent in advanced ultrasound.
Acknowledgement
None.
Conflict of Interest
Authors declare no conflict of interest.
References
- NICE (2017) NICE - Fertility problems: assessment and treatment. Clinical guideline [CG156].
- Campbell S (2019) Ultrasound Evaluation in Female Infertility: Part 1, the Ovary and the Follicle. Obstet Gynecol Clin North Am 46(4): 683-696.
- Practice Committee of the American Society for Reproductive Medicine (2015) Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil Steril 103(3): e9-e17.
- Amanvermez R, Tosun M (s) An update on ovarian aging and ovarian reserve tests. Int J Fertil Steril 9(4): 411-415.
- Coelho Neto MA, Ludwin A, Borrell A, Benacerraf B, Dewailly D, et al. (2018) Counting ovarian antral follicles by ultrasound: a practical guide. Ultrasound Obstet Gynecol 51(1): 10-20.
- Broekmans FJM, de Ziegler D, Howles CM, Gougeon A, Trew G, et al. (2010) The antral follicle count: Practical recommendations for better standardization. Fertil Steril 94(3): 1044-1051.
- Iliodromiti S, Anderson RA, Nelson SM (2015) Technical and performance characteristics of anti-Müllerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update 21(6): 698-710.
- Fleming R, Seifer DB, Frattarelli JL, Ruman J (2015) Assessing ovarian response: antral follicle count versus anti-Müllerian hormone. Reprod Biomed Online 31(4): 486-496.
- Deb S, Campbell BK, Clewes JS, Pincott-Allen C, Raine-Fenning NJ (2013) Intracycle variation in number of antral follicles stratified by size and in endocrine markers of ovarian reserve in women with normal ovulatory menstrual cycles. Ultrasound Obstet Gynecol 41(2): 216-222.
- Tal R, Seifer DB (2017) Ovarian reserve testing: a user’s guide. Am J Obstet Gynecol 217(2): 129-140.
- Tsakos E, Tolikas A, Daniilidis A, Asimakopoulos B (2014) Predictive value of anti-müllerian hormone, follicle-stimulating hormone and antral follicle count on the outcome of ovarian stimulation in women following GnRH-antagonist protocol for IVF/ET. Arch Gynecol Obstet 290(6): 1249–1253.
- Farquhar C, Marjoribanks J, Brown J, Fauser BC, Lethaby A, et al. (2017) Management of ovarian stimulation for IVF: narrative review of evidence provided for World Health Organization guidance. Reprod Biomed Online 35(1): 3-16.
- Syrop CH, Willhoite A, Van Voorhis BJ (1995) Ovarian volume: A novel outcome predictor for assisted reproduction. Fertil Steril 64(6): 1167-1171.
- Zaidi J, Barber J, Kyei-Mensah A, Bekir J, Campbell S, et al. (1996) Relationship of ovarian stromal blood flow at the baseline ultrasound scan to subsequent follicular response in an in vitro fertilization program. Obstet Gynecol 88(5): 779-784.
- Lotti F, Maggi M (2015) Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update 21(1): 56-83.
- Teede H (2018) International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2018. National Health and Medical Research Council (NHMRC).
- Campbell S (2019) Ultrasound Evaluation in Female Infertility: Part 2, the Uterus and Implantation of the Embryo. Obstet Gynecol Clin North Am 46(4): 697-713.
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF (1992) Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum Reprod 7(10): 1342-1346.
- Rosenfield RL, Ehrmann DA (2016) The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocrine Reviews 37(5): 467-520.
- Frattarelli JL, Lauria-Costab DF, Miller BT, Bergh PA, Scott RT (2000) Basal antral follicle number and mean ovarian diameter predict cycle cancellation and ovarian responsiveness in assisted reproductive technology cycles. Fertil Steril 74(3): 512–517.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1): 19-25.
- Agrawal R, Sladkevicius P, Engmann L, Conway GS, Payneet NN, et al. (1998) Serum vascular endothelial growth factor concentrations and ovarian stromal blood flow are increased in women with polycystic ovaries. Hum Reprod 13(3): 651-655.
- Hamdan M, Dunselman G, Li TC, Cheong Y (2015) The impact of endometrioma on IVF/ICSI outcomes: A systematic review and meta-analysis. Hum Reprod Update 21(6): 809-825.
- Dishuck CF, Perchik JD, Porter KK, Gunn DD (2019) Advanced Imaging in Female Infertility. Curr Urol Rep 20(11): 77.
- (2019) Infertility Workup for the Women’s Health Specialist. Obstet Gynecol 133(6): 1294-1295.
- Vickramarajah S, Stewart V, van Ree K, Hemingway AP, Crofton ME, et al. (2017) Subfertility: What the radiologist needs to know. Radiographics 37(5): 1587-1602.
- Levaillant JM, Pasquier M, Massin, N (2019) A novel concept for female infertility exploration: the Fertiliscan©, a dedicated all-in-one 3D ultrasound exploration. J Gynecol Obstet Hum Reprod 48(5): 363-367.
- Jayaprakasan K, Chan Y, Islam R, Haoula Z, Hopkisson J, et al. (2012) Prediction of in vitro fertilization outcome at different antral follicle count thresholds in a prospective cohort of 1,012 women. Fertil Steril 98(3): 657-663.
- Revelli A, Martiny G, Piane LD, Benedetto C, Rinaudo P, et al. (2014) A critical review of bi-dimensional and threedimensional ultrasound techniques to monitor follicle growth: Do they help improving IVF outcome? Reprod Biol Endocrinol 12(1): 1-9.
- Nargund G, Bourne T, Doyle P, Parsons J, Cheng W, et al. (1996) Associations between ultrasound indices of follicular blood flow, oocyte recovery and preimplantation embryo quality. Hum Reprod 11(1): 109-113.
- Van Blerkom J, Antczak M, Schrader R (1997) The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: Association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod 12(5): 1047-1055.
- Robson SJ, Barry M, Norman RJ (2008) Power Doppler assessment of follicle vascularity at the time of oocyte retrieval in in vitro fertilization cycles. Fertil Steril 90(6): 2179-2182.
- Healy MW, Hill MJ, Levens ED (2015) Optimal oocyte retrieval and embryo transfer techniques: Where we are and how we got here. Semin Reprod Med 33(2): 83-91.
- Brinsmead M, Oliver SM, Shumack J, Raymond S, et al. (1989) A randomized trial of laparoscopy and transvaginal ultrasound-directed oocyte pickup for in vitro fertilization. J In Vitro Fert Embryo Transf, 6(3): 149-154.
- Levi-Setti PE, Cirillo F, Scolaro V, Morenghi E, Heilbron F, et al. (2018) Appraisal of clinical complications after 23,827 oocyte retrievals in a large assisted reproductive technology program. Fertil Steril 109(6): 1038-1043.
- Torry DS, Leavenworth J, Chang M, Maheshwari V, Groesch K, et al. (2007) Angiogenesis in implantation. J Assist Reprod Genet 24(7): 303-315.
- Richter KS, Bugge KR, Bromer JG, Levy MJ (2007) Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil Steril 87(1): 53-59.
- Zhao J, Zhang Q, Li Y (2012) The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reprod Biol Endocrinol 10: 100.
- Wang L, Qiao J, Li R, Zhen X, Liu Z (2010) Role of endometrial blood flow assessment with color Doppler energy in predicting pregnancy outcome of IVF-ET cycles. Reprod Biol Endocrinol 8: 122.
- Steer CV, Campbell S, Tan SL, Crafford T, Mills C, et al. (1992) The use of transvaginal color flow imaging after in vitro fertilization to identify optimum uterine conditions before embryo transfer. Fertility and Sterility 57(2): 372-376.
- Somigliana E, Vercellini P, Daguati R, Pasin R, De Giorgi O, et al. (2007) Fibroids and female reproduction: A critical analysis of the evidence. Hum Reprod Update 13(5): 465-476.
- Sunkara SK, Khairy M, El-Toukhy T, Khalaf Y, Coomarasamy A (2010) The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: A systematic review and meta-analysis. Hum Reprod 25(2): 418-429.
- Casini ML, Rossi F, Agostini R, Unfer V (2006) Effects of the position of fibroids on fertility. Gynecol Endocrinol 22(2): 106-109.
- Saravelos SH, Cocksedge KA, Li TC (2008) Prevalence and diagnosis of congenital uterine anomalies in women with reproductive failure: A critical appraisal. Hum Reprod Update 14(5): 415-429.
- Graupera B, Pascual MA, Hereter L, Browne JL, Úbeda B, et al. (2015) Accuracy of three-dimensional ultrasound compared with magnetic resonance imaging in diagnosis of Müllerian duct anomalies using ESHRE-ESGE consensus on the classification of congenital anomalies of the female genital tract. Ultrasound Obstet Gynecol 46(5): 616-622.
- Chan YY, Jayaprakasan K, Tan A, Thornton JG, Coomarasamy A, et al. (2011) Reproductive outcomes in women with congenital uterine anomalies: A systematic review. Ultrasound Obstet Gynecol 38(4): 371-382.
- Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, et al. (2014) Uterine adenomyosis and in vitro fertilization outcome: A systematic review and meta-analysis. Hum Reprod 29(5): 964-977.
- Sallam HN, Sadek SS (2003) Ultrasound-guided embryo transfer: A meta-analysis of randomized controlled trials. Fertil Steril 80(4): 1042-1046.
- Ata B, Tulandi T (2011) Ultrasound automated volume calculation in reproduction and in pregnancy. Fertil Steril 95(7): 2163-2170.
- Jayaprakasan K, Hilwah N, Kendall NR, Hopkisson JF, Campbellet BK, et al. (2007) Does 3D ultrasound offer any advantage in the pretreatment assessment of ovarian reserve and prediction of outcome after assisted reproduction treatment? Hum Reprod 22(7): 1932-1941.
- Raine-Fenning N, Jayaprakasan K, Chamberlain S, Devlin L, Priddle H, et al. (2009) Automated measurements of follicle diameter: a chance to standardize? Fertil Steril 91(4 Suppl)1469-1472.
-
Tara Giacchino, Rebecca Karkia, Hasib Ahmed. State-Of-The-Art Ultrasound in the Management of the Infertile Couple. W J Gynecol Women’s Health. 4(3): 2020. WJGWH.MS.ID.000590.
Female infertility, Male infertility, Ultrasound, 3D imaging, Ovarian reserve, Assisted reproductive technology, Oocyte quality, Oocyte retrieval, Embryo implantation, Embryo transfer
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






