 Mini Review
Mini Review
Raman Fluorescent Technologies in Stomatology
Aleskandrov MT*, Nikiforova ED Ahmetov Ali Asker, Artemova OA, Razumova SN and Namiot ED
Russian Ministry of Health, Russia
Aleskandrov MT, Russian Ministry of Health, Russia.
Received Date: June 29, 2019; Published Date: July 11, 2019
Abstract
An experimental theoretical substantiation and hardware and software application of Raman-fluorescent medical technologies in dentistry are presented. Highly sensitive and highly specific express methods for diagnosing caries, the degree of demineralization of hard tooth tissues, specific indication of microbes and rapid determination of their sensitivity to drugs, verification of drugs and narcotic substances, express diagnosis of benign and malignant tumors are proposed. A wide range of clinical and diagnostic capabilities of the method and the proposed domestic hardwaresoftware complex of Raman-fluorescent diagnostics is recommended for clinical use.
Keywords: Raman fluorescent diagnostics; Hardware software complex; Caries; Raman line; Hydroxyl appatite tooth tissue; Raman spectra of microbes; drugs; Alcohols; Narcotic drugs; Raman fluorescence tumors
Introduction
Currently, in medicine, including in dentistry, laser innovative, express medical treatment and diagnostic technologies based on the use of raman and/or luminescent hardware-software complexes are becoming increasingly important. Probe laser radiation (in a wide spectral and energy range) is used as a means of treatment [1-4] and at the same time as a means of exciting raman-luminescent diagnostic characteristics [5] of the studied biological object (the proposed study is devoted to this area of biophotometry). The technique is based on the fact that according to the registered Raman and / or luminescent spectral (LRFD) characteristics of the objects under study (metabolites of microbes, cells, tissues, biological fluids in normal conditions and in pathology) it is possible to diagnose diseases and processes of microbial, metabolic, neoplastic and other nature [6-7].
On the basis of LRFD, the In Spectr M medical hardwaresoftware complex of the same name was developed and released [Patent for utility model RU 130700 dated July 27, 2013]. It helps to record the spectral position and relative intensities of Raman and/ or luminescent lines-a kind of “fingerprints” of the substance under investigation, search and compare them with the spectral database and then identify the object under study. The analysis results are processed using a convenient software interface and can be easily recorded in the “PNG” format. The analysis can be carried out on the In Spectr M532 Raman microscope (Figure 1). This modification is necessary when working with samples of microscopic size outside the mouth (scraping, smear, imprint of oral tissues; detritus from the root canal of the tooth), samples with heterogeneous and complex surface (granulation tissue of the periodontal pocket, biopsy material-tumor).

For a visual study of the object, a standard microscope operating mode using eyepieces and / or a video camera is used. Its design uses a 30mw blue or green single mode laser, which provides highquality spectral resolution. In the manual mode, the focus is selected on a specific point of the sample, in which the LRFD spectrum is recorded. From one object, it is advisable to remove 8-10 indicators at different points with their subsequent averaging and outputting on one graph for analysis. It is also possible to use LRFD directly in the oral cavity due to the compactness and portability of the modification of the In Spectr M device, which includes both optical fiber and various nozzles (Figure 2).

Results of the research of the range of clinical application of raman-fluorescent medical technologies
All studies and clinical observations were performed on the same hardware-software complex - APC RFD; they completely satisfy the metrological parameters (according to analytical and diagnostic sensitivity and specificity exceeds the existing analogues by 10x4-10x6 times the patent for the invention RU 2526584 from 27.08.2014, the patent for the invention RU 2543691 from 10.03.2015], and the needs of clinical dentistry (certificate from 06.2011 No. 6361 and from May 18, 2015 No. RZN 2015 \ 2419) in the conditions of mass patient reception.
Research of hard tissues of zuba
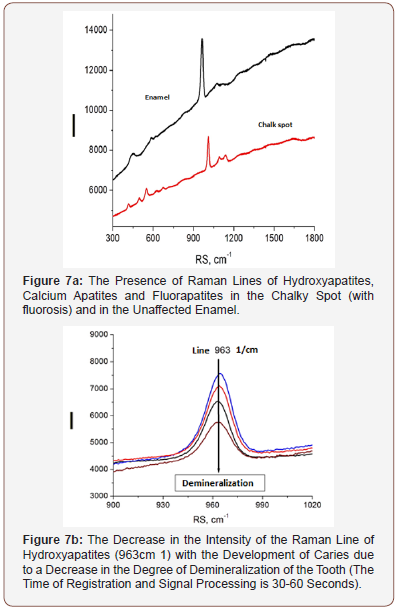
APK and the RFD technique, thanks to its technical characteristics, made it possible to study a wide range of its clinical use (possibilities and prospects). In particular, the selection of the most informative AIC for the diagnosis of caries was carried out (Figures 3-5). And the spectra of RFD of caries and the degree of demineralization of the tooth were studied-measuring time 10-20 seconds (Figures 6,7).
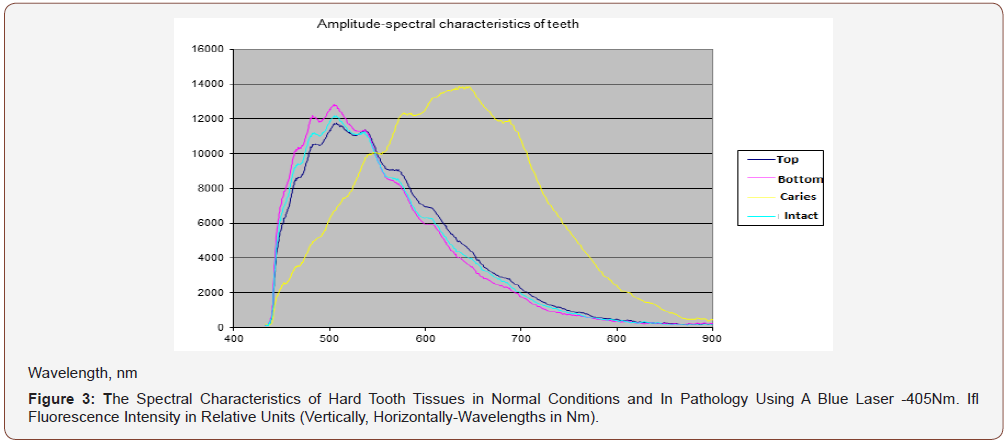
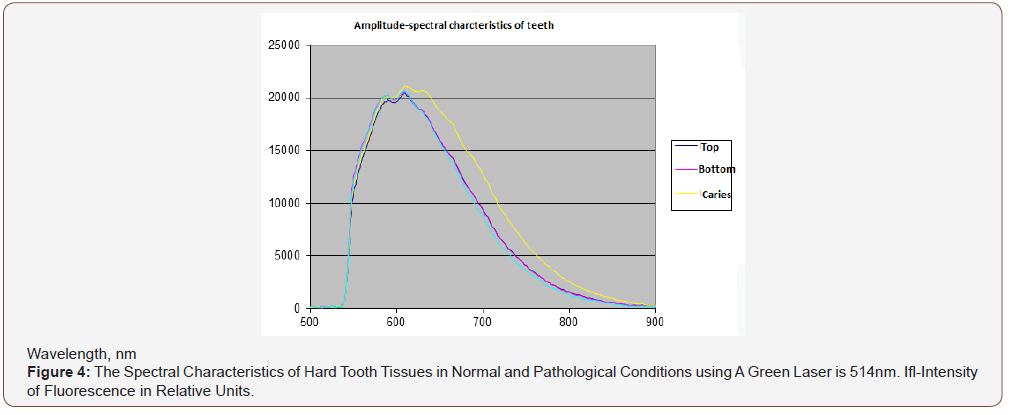
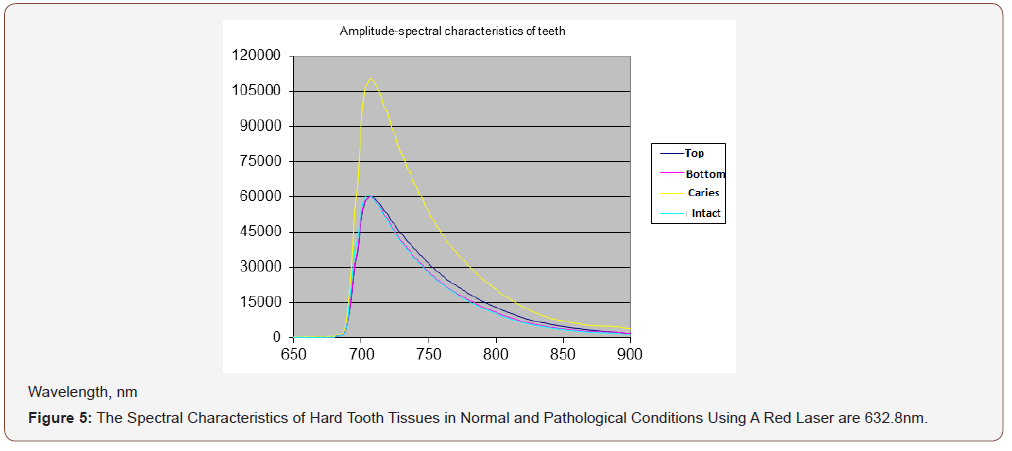
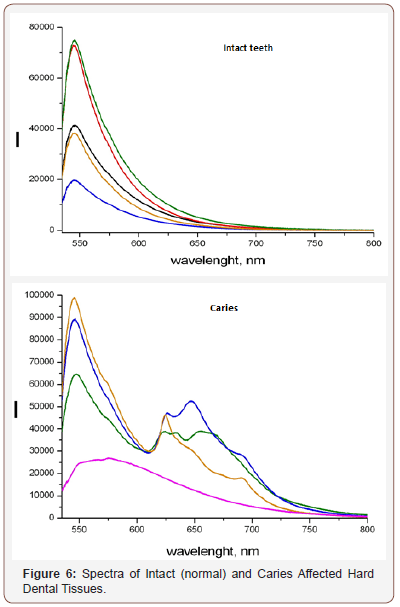
From the presented data (Figures 7a,b), on the chalky stains of teeth (with fluorosis) lines of fluorapatites are seen in the spectrum of scattered light, the content of which is greatly increased. On healthy teeth, the dominant lines of hydroxyapatite and calcium apatite are visible. Significant differences in the spectra of intact and carious tissues of the tooth appear (Figures 3-6). In the range of 620-720nm, in the spectra of the second, luminescent lines of bacterial microflora (spectrum shift to the right) are clearly visible, which are absent in intact tissues. Obviously, the RFD technique is pathogenetically justified, since in the express mode, it is able to identify such pathogenesis of caries as a microbial factor (presented below), as well as the degree of demineralization of the tooth under the influence of this factor, which radically distinguishes it from other, much less informative methods [6].
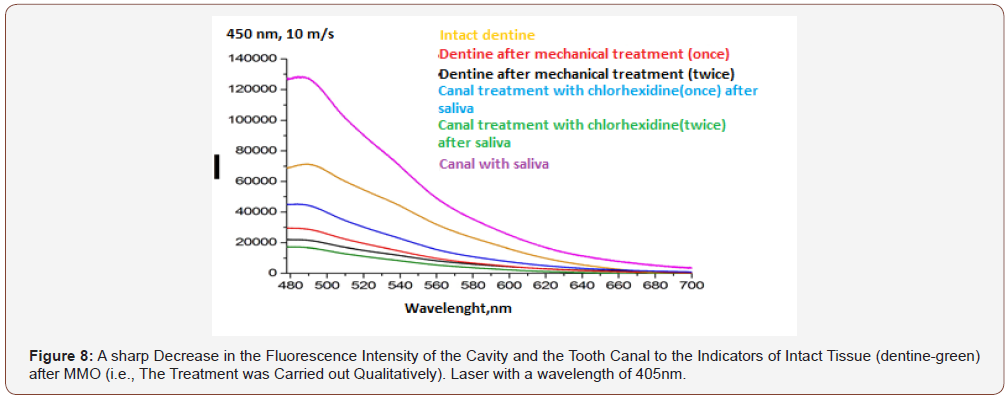
RFD technology makes it possible to objectively and qualitatively evaluate the effectiveness of mechanical and drug treatment of the cavity (MMO) and the tooth canal (Figure 8), the state of oral hygiene (Figure 9) with a high degree of analytical sensitivity (Figure 12) that is not inferior to the bacteriological method ( 10x4-10x5 CFU/ml).
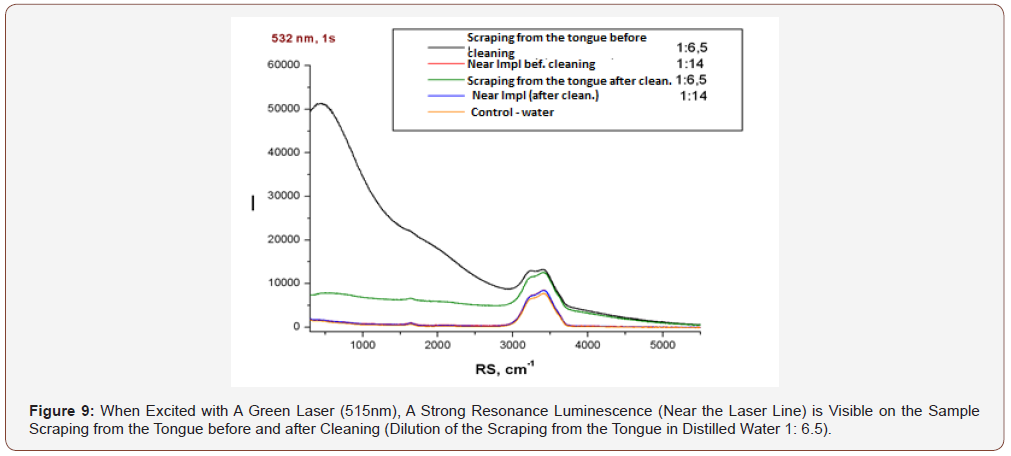

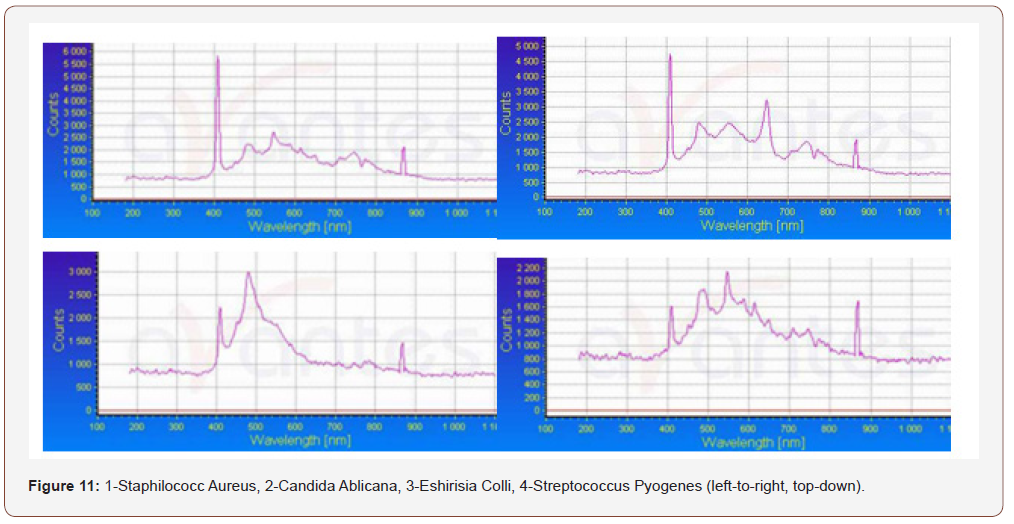
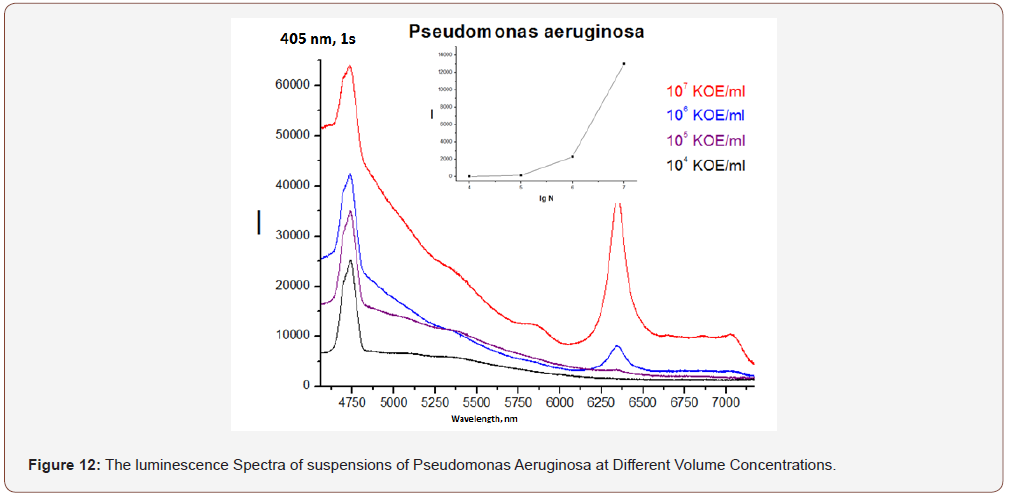
As can be seen from (Figure 11), the luminescence intensity of the scraping from the tongue to its purification is an order of magnitude greater than that after (even when diluting the scraping in water 1: 6,5). There are no differences between the samples near Impl (dental implant) before and after cleaning (dilution 1:14), but their luminescence is more intense in both cases than in the control water sample.
Study of microbial factor and The Possibility of Express to determine its sensitivity to antimicrobial preparations (Express mode)
(Figure 10) On the left, a comparison of the spectra of Pseudomonas aeruginosa and Staphylococcus aureus; on the right is a comparison of the spectra of different clinical strains of Staphylococcus aureus. Thus, the proposed method can apparently be used to indicate and differentiate microbes according to their specific RFD spectra (after working on their database).
Indication of drugs and narcotic substances
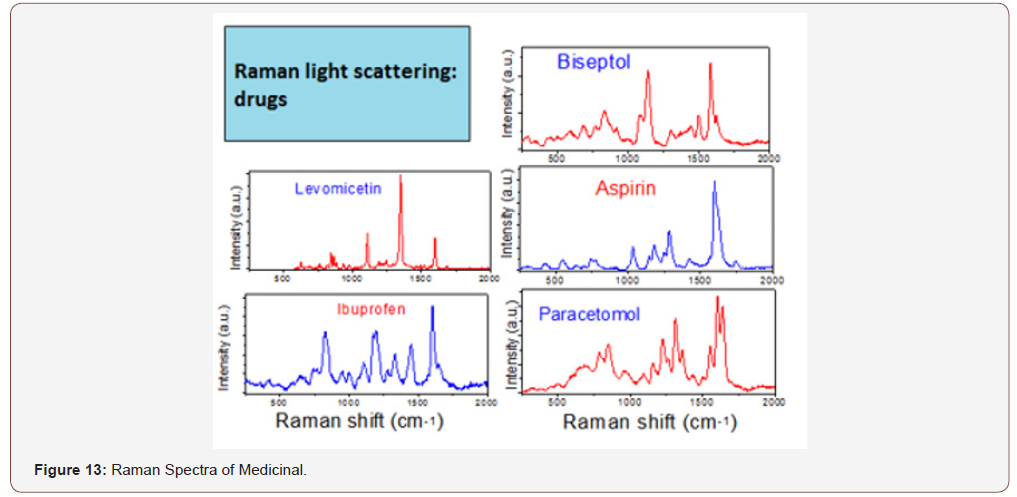
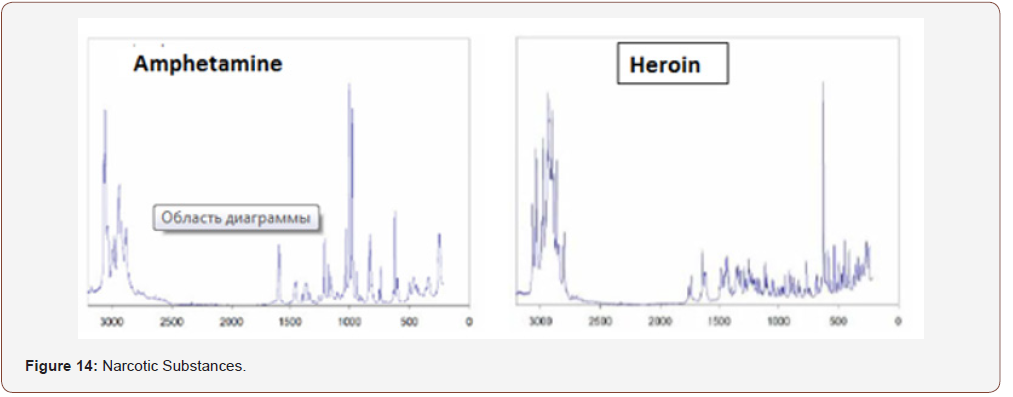
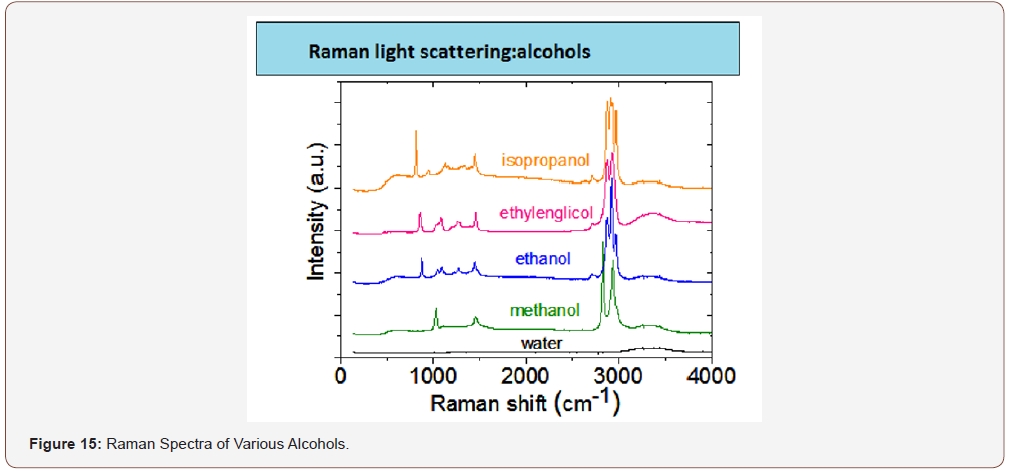
The concept of the use of RFD in dentistry and other areas of medicine is not only more informative, convenient and small. On the basis of this technique, it is possible to identify various organic (drugs, alcohols, narcotic substances) and inorganic (see above) substances, the database of which will exclude the acquisition and use of fakes and non-certified products. you only need to purchase a database of the product and enter it into the memory used for the clinical purposes of the computer (Figures 13-15).
Principles of determining the sensitivity of microbes to antimicrobial preparations
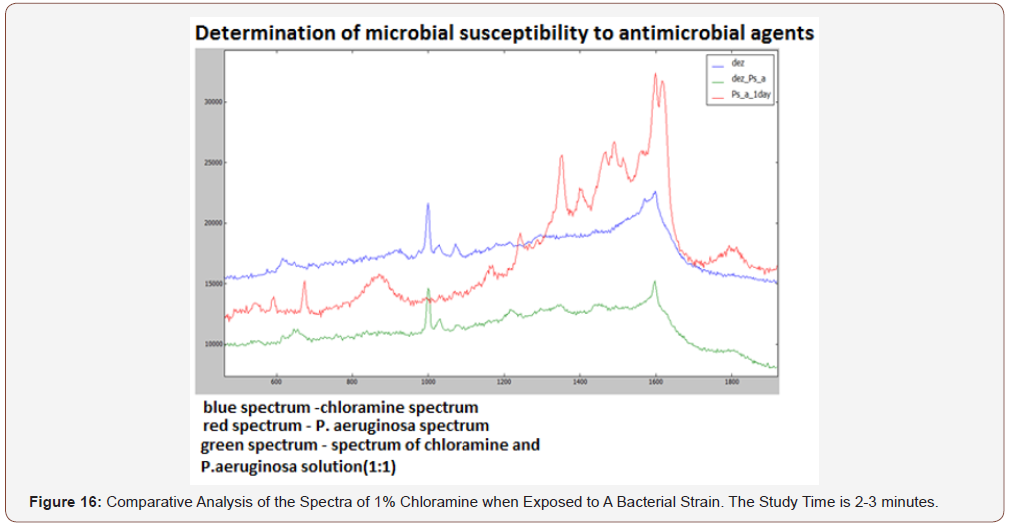
(Figure 16) Comparative analysis of the spectra of 1% chloramine when exposed to a bacterial strain. The study time is 2-3 minutes. After the introduction of chloramine, the spectrum of the pyocyanic stick disappeared and the spectrum of the solution of chloramine+bacteria (1: 1) became similar to the spectrum of chloramine (Figure 9). This means that the bacteria under the action of this antiseptic are inactivated and destroyed. This makes it possible to individually select an adequate etiotropic therapy in the complex treatment of patients, and in combination with the above mentioned LRFD technologies, to evaluate the effectiveness of the treatment as a result.
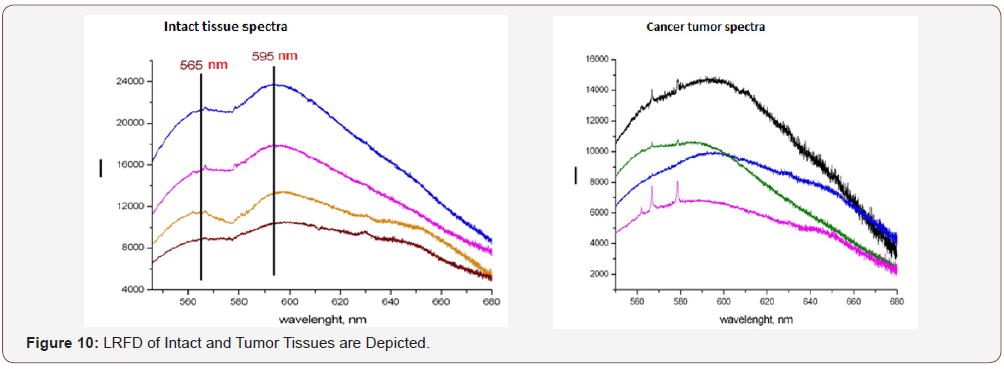
Principles of express differentiation and determination (seconds) of intact and tumor tissues
In the concept of LRFD technology development, it became possible to expressly determine tissue belonging, to differentiate between intact tissues, benign and malignant tumors (Figure 10). Thus, LRFD medical technology is a unique tool in the arsenal of a dentist. It combines expressivity, high diagnostic and, most importantly, analytical sensitivity, not inferior to modern methods (bacteriological, histological, optical, etc.) with the functions of high-tech laboratory equipment [8]. The method and equipment of the APC RFD, for its implementation, allows rapid identification of tissues and substances of organic and inorganic nature directly at the workplace of a dentist, expanding diagnostic capabilities in relation to diseases and processes of microbial nature, neoplastic processes of tissues and organs of the maxillofacial region in normal and pathology. At the same time, it fully meets the needs of mass dental reception, allowing you to analyze material from the oral cavity both directly in it (using optical fiber) and outside it based on focusing the laser beam using a microscope combined with a laser device. Medical technology requires its early implementation in clinical practice.
Acknowledgement
None.
Conflict of Interest
No conflicts of interest.
References
- Aleksandrov MT, Zubov SV, Berezinskaya AC (2013) Experimental and theoretical substantiation of the principles and features of the application of the method of laser-conversion diagnostics for assessing the state of hard tooth tissues in normal and pathological conditions (caries). Russian Dental Journal p. 6-10.
- Aleksandrov MT, Zuev VM, Kukushkin (2013) VI Study of the spectral characteristics of the pelvic organs in women and their clinical significance. Oncogynecology p. 61-67.
- Aleksandrov MT, Kukushkin VI, Ambartsumyan OA (2013) Identification of microorganisms based on the effect of giant Raman scattering. Journal of Microbiology, Epidemiology and Immunobiology p. 97-100.
- Aleksandrov MT (2008) Laser clinical biophotometry (theory, experiment, practice). P. 584.
- Kukushkin VI, Vankov AB, Kukushkin IV (2013) Interrelation of giant amplification of Raman scattering signals and luminescence on nanostructured metal surfaces. Letters to Zhetf 98: 383-388.
- Zaitseva EV (2000) Development of a method for fluorescent diagnosis of hard tooth tissues with carious lesions: author. Diss Cand Med Sciences p. 10.
- Sarycheva IN, Yanushevich OO, Minakov DA (2012) Early diagnosis of dental caries using laser-induced fluorescence. Russian dentistry pp. 47-58.
- Filatov MV (2004) The use of laser fluorescence to assess the hygienic condition of the oral cavity. Diss Cand med sciences p. 120.
-
Aleskandrov MT, Nikiforova ED Ahmetov Ali Asker, Artemova OA, Razumova SN, Namiot ED. Raman Fluorescent Technologies in Stomatology. On J Dent & Oral Health. 2(2): 2019. OJDOH.MS.ID.000535.
-
Metrological parameters, Staphylococcus aureus, Narcotic substances, Pathogenesis, Hydroxyapatite, Dentistry, Drugs, Malignant tumors
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






