 Case Report
Case Report
Antisynthetase Syndrome with Anti-Pl12 Antibodies and Cardiac Involvement
Ibtissam Romani*, Mohammed El Jamili, Rida Chniber and Mustapha El Hattaoui
Department of Cardiology, Morocco
Ibtissam Romani, Department of Cardiology, University Hospital Mohammed VI, Morocco.
Received Date: February 07, 2019; Published Date: March 27, 2019
Abstract
Background: The antisynthetase syndrome (ASS) is a rare chronic autoimmune disorder associated with a various manifestation including myositis, interstitial lung disease, fever, Raynaud’s phenomenon, “mechanic’s hands, polyarthritis and antibody specificity. Cardiac involvement is relatively rare but possible. We report a case of ASS anti PL12 positive with cardiac manifestations.
Case report: A 36 year old woman was admitted to the Department of Cardiology in our hospital for presyncope episodes, and exertional dyspnea. Physical examination revealed signs of congestive heart failure. Her heart rate was 40 bpm with a second-degree AV block Mobitz I on the electrocardiography. Transthoracic echocardiography showed cardiomyopathy with severe biventricular dysfunction and pulmonary hypertension. Further investigations were done to identify the cause of this cardiomyopathy especially as the patient presented arthralgias for last 2 years and history suggestive of Raynaud’s phenomenon and weakness of limbs. They revealed ASS syndrome with anti PL12 antibodies, diffuse interstitial lung involvement and cardiac involvement. The patient was treated with diuretic and corticosteroid therapy (methylprednisolone 1g pulse). On 4th day of admission, patient developed a complete AV block. Permanent dual chamber pacemaker implantation was performed. The patient was initiated on Cyclophosphamide and oral prednisolone which resulted in improved muscle strength and exercise tolerance.
Conclusion: Antisynthetase syndrome is a rare inflammatory myopathy. The cardiac involvement is even rarer and it is associated with worse outcome. The cardiovascular manifestations are various. This is the first report of ASS associated with complete heart block, cardiomyopathy and pulmonary hypertension in the same patient. Given the possible severe consequences, the cardiac involvement should be considered in patients with antisynthetase syndrome and clinicians should be familiar with this entity.
Keywords: Antisynthetase syndrome; Complete heart block; Cardiomyopathy; Heart failure; Pulmonary hypertension
Abbreviations: ASS: Antisynthetase Syndrome; AV block: Atrioventricular Block; ILD: Interstitial Lung Disease
Introduction
Antisynthetase syndrome (ASS) is a rare, chronic, autoimmune disorder of unknown etiology. It belongs to the category of autoimmune inflammatory myopathies and is characterized by the presence of various but mutually exclusive anti-tRNA synthetase autoantibodies including anti-PL-12 [1,2]. Cardiac involvement is much less reported than in other myopathies and is associated with worse outcomes compared with cases without cardiac involvement [3-6]. Abnormalities of nearly every component of the cardiac structure have been reported, including the pericardium (pericarditis), myocardium (conduction system abnormalities, myocarditis, cardiomyopathy), and endocardium (mitral valve prolapse) [5,6]. It includes subclinical changes or different features like congestive heart failure [7,8]. Although, several reports have described disturbances of rhythm and conduction associated with idiopathic inflammatory myopathies, there are a very few reports in the literature presenting patients with ASS and complete or high grade atrioventricular (AV) blocks. Furthermore, ASS is not typically associated with cardiomyopathy and myocardial involvement in this condition is poorly described. The rare association of complete AV block, cardiomyopathy and pulmonary hypertension with ASS and interesting course of the patient prompted us to report this case.
Case Report
A 36 year old woman was admitted to the Cardiology Department in Mohamed VI Hospital of Marrakech on June 2017 due to generalized weakness, presyncope episodes, and exertional dyspnea (NYHA class III).
Past medical history revealed presence of arthralgias for last 2 years involving bilateral interphalangeal and metacarpophalangeal joints, elbow and knee joints, history suggestive of Raynaud’s phenomenon and weakness of limbs. She had no known allergies and family history was not significant. She did not use alcohol, tobacco, illicit substances, or supplements. At presentation, she appeared fatigued and dyspneic after speaking a few sentences. Physical examination revealed jugular vein distension and peripheral edema. Auscultation of chest revealed basal inspiratory crepitations. Her heart rate was 40 bpm and the blood pressure was 100/55 mmHg. In addition, she had “mechanic’s hands” and marked bilateral proximal muscle weakness of the upper and lower extremities. The Electrocardiography (ECG) revealed a type 1 second-degree AV block Mobitz I.
Transthoracic echocardiography (TTE) showed severe enlargement of all 4 cardiac chambers, left ventricular end-diastolic diameter (LVEDD) of 60 cm, left ventricular ejection fraction (LVEF) of 30%–35%, marked right ventricular (RV) dysfunction, pulmonary hypertension (estimated at 70mmHg) and moderate tricuspid regurgitation, without evidence of pericardial effusion. Laboratory studies showed elevations of serum creatine phosphokinase (CK): 1070 UI/l (Normal<179 UI/l) and lactate dehydrogenase (LDH): 435 UI/l (N = 76–156) and raised CRP: 60mg/l and sedimentation rate: 80 mm/h. Anti–PL12 antibodies was positive. Antinuclear antibody and anti-DNA were negative and other biochemical parameters were normal.
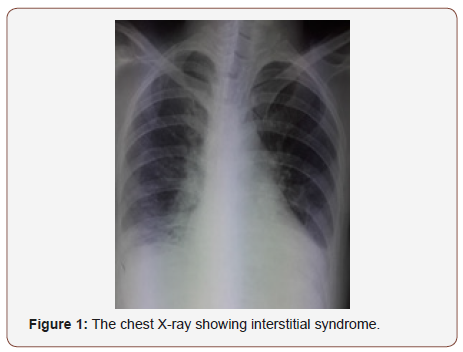
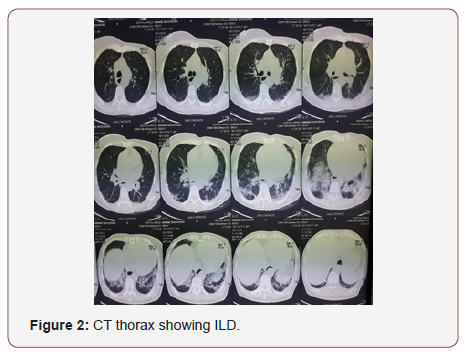
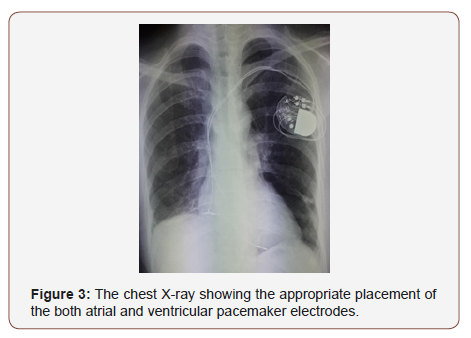
Chest radiograph showed interstitial syndrome (Figure 1). CT scan of thorax revealed signs of diffuse interstitial lung disease (ILD) (Figure 2). Pulmonary function tests were not done. Electromyography (EMG) of all 4 limbs revealed a generalized myopathic pattern. Muscle biopsy from biceps showed inflammatory myopathy consistent with polymyositis. 24-hour Holter recordings showed 2/1 AV block and some ventricular extrasystoles. The positive titer of anti PL12 antibodies associated with myositis supported the diagnosis of ASS with an ILD and cardiac involvement: heart AV block, cardiomyopathy and pulmonary artery hypertension. The patient was treated with diuretic therapy and IV methylprednisolone 1g pulse was started from 3rd day of admission. On 4th day of admission, patient had worsening of symptoms. She had BP of 95/60 mmHg and pulse was 19/min. ECG showed a complete AV block with escape ventricular beats. Due to her clinical presentation, permanent dual chamber pacemaker implantation was performed with no complication (Figure 1-3).
The patient was initiated on Cyclophosphamide pulse, which resulted in improved muscle strength and exercise tolerance during her hospital admission. She was initiated on guidelinebased heart failure management, including angiotensin-converting enzyme inhibitor (ACE-I) and aldosterone antagonist therapies; beta-blocker therapy was initiated before discharge. Patient was discharged on 20th day with no symptoms and improved muscle power and was advised to continue monthly cyclophosphamide and oral prednisolone. She was on regular follow-up with gradual tapering of the steroid. Eight months later, she presented with palpitations. ECG showed atrial flutter reduced with successful electrical cardioversion. Now, at 21 months of follow-up, the patient improved clinically, LVEF improved to of 40%–45%, and pulmonary pressure to 40 mmhg.
Discussion
The inflammatory myopathies comprise a heterogenous group of chronic autoimmune disorders of unknown etiology. Polymyositis (PM) and dermatomyositis (DM) are the two major entities which constitute this group of diseases. As other systemic autoimmune disorders, PM and DM are associated with serum autoantibodies, some of which are detected almost exclusively in these diseases. Several of these autoantibodies are related to particular clinical manifestations, marking, therefore, important clinical subgroups [9]. A major subgroup in inflammatory muscle disease is the ASS characterized by the presence of anti-synthetase antibodies. The presence of AS antibodies along with a distinctive clinical phenotype characterized by inflammatory myopathy, non-erosive arthritis, ILD, fever, and fissured, hyperkeratotic skin changes on the lateral and palmar surface of the hands and fingers (“mechanic’s hands”) constitutes the ASS [10-14]. In patients with AS syndrome, significant morbidity and mortality is attributed to ILD, and the presence of ASS antibodies is the strongest predictor for the development of ILD [14,15].
Different anti-ARS specificities have been described, anti-Jo1 antibodies being the most common. The other antibody specificities, including anti-alanyl (PL12) are less commonly found. Clinically manifest cardiac involvement in PM and DM is relatively rare. In contrast, subclinical manifestations are frequently reported and are predominated by conduction abnormalities and arrhythmias detected by ECG [16]. Cardiovascular manifestations are much less reported in AS syndrome than in other myopathies and are associated with worse outcomes compared with cases without cardiac involvement [3]. Abnormalities of nearly every component of the cardiac structure, including the pericardium (pericarditis), myocardium (conduction system abnormalities, myocarditis, cardiomyopathy, congestive heart failure) have been reported in PM/DM cases, but exact prevalence in ASS is unknown [16,17]. We present a case of typical AS syndrome with polyargtralgia, Raynaud’s phenomenon, ILD and positive anti-PL12 antibody, associated with cardiac involvement represented by clinical heart failure and complete AV block. The young age of our patient, the absence of cardiac past medical history and other etiology, connect the heart block and cardiomyopathy to the AS syndrome. To our knowledge, this association of AS syndrome with 2 cardiac abnormalities in the same patient has not been described in the literature. Complete heart block fortunately occurs very rarely and has been reported in only 2 cases of AS syndrome patients so far.
Proposed mechanisms of cardiac involvement in ASS include coronary artery inflammation leading to vasculitis, intimal proliferation, microvascular disease, or coronary vasospasm, all of which may contribute to impaired LV function and conduction abnormalities [18,19]. It is also probable that underlying autoimmune processes may contribute to myocarditis owing to its effects on humoral and cellular immune mechanisms [20]. Cardiac involvement in inflammatory myopathies is polymorphic. The most frequently reported clinically overt manifestations are congestive heart failure, cardiomyopathy, right heart failure, myocarditis, pulmonary hypertension (PH) and conduction abnormalities [21- 24]. Pericardial effusion also reported in one case [25]. Myocarditis can indeed lead to the development of dilated cardiomyopathy in 30% of cases. In inflammatory myositis and in ASS in particular, the occurrence of PH has never been systematically evaluated and its description rests only upon isolated case reports [26,27]. PH comprises many causes, including pulmonary arterial hypertension (PAH), left heart disease, chronic lung diseases and others. Overt cardiac manifestations may be apparent at time of myositis diagnosis but may also develop and become manifest after the treatment has been initiated. Cardiac failure usually occurs together with active skeletal muscle involvement, but it may also develop despite low inflammatory activity in skeletal muscle disease, during immunosuppressive treatment or even when in remission.
As expected, there are no guidelines to aid in the diagnosis of cardiac manifestations associated with ASS. ECG is a reasonable initial test to screen for cardiac involvement. Echocardiography is helpful for the detection of cardiomyopathy and PH. However, gadolinium-enhanced cardiac MRI is more useful for the diagnosis of myocarditis, because it can show areas of delayed enhancement, consistent with an inflammatory process [28]. Corticosteroid therapy remains the first line of treatment for ASS, although there are no controlled studies of this therapy [29]. Steroid-sparing immunosuppressant agents, including azathioprine, mycophenolate mofetil, cyclosporine, and tacrolimus can be used; however, only case reports are available on the use of these treatments for ASS, and their effects on ASS–associated cardiac involvement are unknown [29]. Medical therapy does not necessarily prevent cardiac complications, such as complete heart block or fatal arrhythmias, which have occurred in patients receiving treatment with steroids or steroid-sparing agents [30-32]. The effects of corticosteroid treatment and other immunosuppressive on cardiac manifestations are conflicting. In some cases, congestive heart failure improved during corticosteroid treatment but progressed in other individuals [33,34]. The conductive disorders are generally unresponsive to corticosteroids [32]. Most authors agree on the need for pacemaker implantation in symptomatic conduction disorders as is the case of our patient [1-4]. In the autopsy study by Den bow et al. [35], the occurrence of myocarditis appeared to be independent of steroid therapy [34]. Besides the immunosuppressive therapy, patients with congestive heart failure have been treated with traditional heart medication. Recently there was one report of successful heart transplant for dilated cardiomyopathy in a woman with polymyositis, with a 14-month post-operative observation period [36].
Conclusion
In conclusion, this case report emphasizes that cardiac involvement in ASS in particular complete heart block, pulmonary hypertension, cardiomyopathy and resultant congestive heart failure is possible and may have very serious clinical implications. Based on this observation, we suggest that ASS should be considered in patients presenting with an idiopathic cardiomyopathy, conduction abnormalities and inflammatory myopathy. Finally, further investigation is needed to elucidate the pathophysiologic mechanisms of ASS to improve diagnostic and therapeutic strategies.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Marguerie C, Bunn CC, Beynon HL, Bernstein RM, Hughes JM, et al. (1990) Polymyositis, pulmonary fibrosis and autoantibodies to aminoacyl-tRNA synthetase enzymes. Q J Med 77: 1019-1038.
- Hervier B, Devilliers H, Stanciu R, Meyer A, Uzunhan Y, et al. (2012) Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev 12: 210-217.
- Oppenheim H (1899) Zur dermatomyositis. Ber Klin Wochenschr 36: 805-807.
- Bazzani C, Cavazzana I, Ceribelli A, Vizzardi E, Dei Cas L, et al. (2010) Cardiological features in idiopathic inflammatory myopathies. J Cardiovasc Med 11: 906-911.
- Hochberg MC, Feldman D, Stevens MB (1986) Adult onset polymyositis/- dermatomyositis: an analysis of clinical and laboratory features and survival in 76 patients with a review of the literature. Semin Arthritis Rheum 15: 168-178.
- Zhang L, Wang GC, Ma L, Zu N (2012) Cardiac Involvement in adult polymyositis or dermatomyositis: a systematic review. Clin Cardiol 35: 686- 691.
- Lundberg IE (2006) The heart in dermatomyositis and polymyositis. Rheumatology 45: iv18-iv21.
- Van Gelder H, Charles-Schoeman C (2014) The heart in inflammatory myopathies. Rheum Dis Clin North Am 40: 1-10.
- Medsger TA, Oddis CV (1994) Inflammatory muscle disease. Clinical features. In: Rheumatology. JH Klippel and PA Dieppe. Mosby Eds London, UK, pp. 6.12.1-6.12.14.
- Yoshida S, Akizuki M, Mimori T, Yamagata H, Inada S, et al. (1983) The precipitating antibody to an acidic nuclear protein antigen, the Jo-1, in connective tissue diseases. A marker for a subset of poly-myositis with interstitial pulmonary fibrosis. Arthritis Rheum 26: 604-611.
- Targoff IN (2008) Autoantibodies and their significance in myositis. Curr Rheumatol Rep 10: 333-340.
- Targoff IN (2002) Laboratory testing in the diagnosis and management of idiopathic inflammatory myopathies. Rheum Dis Clin North Am 28: 859-890.
- Brouwer R, Hengstman GJ, Vree Egberts W, Ehrfeld H, Bozic B, et al. (2001) Autoantibody profiles in the sera of European patients with myositis. Ann Rheum Dis 60: 116-123.
- Katzap E, Barilla-LaBarca ML, Marder G (2011) Antisynthetase syndrome. Curr Rheumatol Rep 13: 175-181.
- Vancsa A, Csipo I, Nemeth J, Devenyi K, Gergely L, et al. (2009) Characteristics of interstitial lung disease in SS-A positive/Jo-1 positive inflammatory myopathy patients. Rheumatol Int 29: 989-994.
- Hochberg MC, Feldman D, Stevens MB (1986) Adult onset polymyositis/- dermatomyositis: an analysis of clinical and laboratory features and survival in 76 patients with a review of the literature. Semin Arthritis Rheum 15: 168-178.
- NilomKhound, MrinalCh, Bhattacharyya, BhaskarJyotiKakati (2018) Anti Synthetase Syndrome – A Case Report, IOSR Journal of Dental and Medical Sciences 2279-0861.
- Haupt HM, Hutchins GM (1982) The heart and cardiac conduction system in polymyositis-dermatomyositis: a clinicopathologic study of 16 autopsied patients. Am J Cardiol 50: 998-1006.
- Marder G, Greenwald R (2009) Antisynthetase syndrome. In: Kagen LJ (ed.), The inflammatory myopathies New York Humana Press USA, pp. 191-206.
- Ghirardello A, Zampieri S, Tarricone E, Iaccarino L, Bendo R, et al. (2006) Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity 39: 217-221.
- Poveda Gomez F, Merino JL, Mate I, Sobrino JA, Camacho J, et al. (1993) Polymyositis associated with anti-Jo1 antibodies: severe cardiac involvement as initial manifestation. Am J Med 94: 110-111.
- Ketlogetswe KS, Aoki J, Traill TA, Cingolani OH (2011) Severe aortic regurgitation secondary to antisynthetase syndrome. Circulation 124: e40-e41.
- Kavita Sharma, Ana-Maria Orbai, Dipan Desai, Oscar H Cingolani, Marc K Halushka, et al. (2014) Brief report: Antisynthetase Syndrome- Associated Myocarditis. J Card fail 20(12): 939-945.
- Aditya Shaha, Samir R Patel (2016) Acute Onset Anti-Synthetase Syndrome. J Clin Med Res 8(9): 683-687.
- Bazzani C, Cavazzana I, Ceribelli A, Vizzardi E, Dei Cas L, et al. (2010) Cardiological features in idiopathic inflammatory myopathies. J Cardiovasc Med 11: 906-911.
- OAMinai (2009) Pulmonary hypertension in polymyositisdermatomyositis: clinical and hemodynamic characteristics and response to vasoactive therapy. Lupus 18: 1006-1010.
- S Chatterjee, C Farver (2010) Severe pulmonary hypertension in Anti- Jo-1 syndrome. Arthritis Care Res (Hoboken) 62: 425-429.
- Ohata S, Shimada T, Shimizu H, Murakami Y, Matsuno Y, et al. (2002) Myocarditis associated with polymyositis diagnosed by gadolinium- DTPA enhanced magnetic resonance imaging. J Rheumatol 29: 861-862.
- Katzap E, Barilla-LaBarca ML, Marder G (2011) Antisynthetase syndrome. Curr Rheumatol Rep 13: 175-181.
- White PG, Podgorski MR, McLeod TI, Clarke AK (1988) Acute myocarditis causing fatal ventricular arrhythmia in treated polymyositis. Br J Rheumatol 27: 399-402.
- Allanore Y, Vignaux O, Arnaud L, Puéchal X, Duboc D, et al. (2006) Effects of corticosteroids and immunosuppressors on idiopathic inflammatory myopathy related myocarditis evaluated by magnetic resonance imaging. Ann Rheum Dis 65: 249-252.
- Younes M, Béjia I, Zrour-Hassen S, Touzi M, Haddada F, et al. (2005) Polymyositis with antisynthetase syndrome and cardiac involvement. Rev Med Interne 26: 673-675.
- Oka M, Raasakka T (1978) Cardiac involvement in polymyositis. Scand J Rheumatol 7: 203-208.
- Stern R, Godbold JH, Chess Q, Kagen LJ (1984) ECG abnormalities in Polymyositis. Arch Intern Med 144: 2185-2189.
- Denbow CE, Lie JT, Tancredi RG, Bunch TW (1979) Cardiac involvement in polymyositis. Arthritis Rheum 22: 1088-1092.
- Afzal A, Higgins RSD, Philbin EF (1999) Heart transplant for dilated cardiomyopathy associated with polymyositis. Heart 82: e4.
-
Ibtissam Romani, Mohammed El Jamili, Rida Chniber, Mustapha El Hattaoui. Antisynthetase Syndrome with Anti-Pl12 Antibodies and Cardiac Involvement. On J Cardio Res & Rep. 1(4): 2019. OJCRR.MS.ID.000518.
-
Antisynthetase syndrome, Complete heart block, Cardiomyopathy, Heart failure, Pulmonary hypertension, Interstitial lung disease, Cyclophosphamide, Etiology, Endocardium, Metacarpophalangeal joints, Lactate dehydrogenase, Transthoracic echocardiography.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






