 Research Article
Research Article
Simulation of Adiabatic Gasification of Corn Straw using Air-Steam Blends as an Oxidizing Agent
Galindo José, Gamba Iván, Amaris María and Bonilla Javier*
Grupo de Investigación de Aprovechamiento Tecnológico de Materiales y Energía, Colombia
Bonilla Javier, Grupo de Investigación de Aprovechamiento Tecnológico de Materiales y Energía, GIATME, Universidad ECCI, Bogotá, Colombia.
Received Date: January 10, 2019; Published Date: February 05, 2019
Abstract
Due to the increase of consumption in the world of energy and the pollution caused by fossil fuel combustion processes, it is necessary to generate new technologies for the use of alternative fuels, allowing to reduce the dependence on fossil fuels such as oil, gas and coal. In general, the combustion processes of fossil fuels produce greenhouse gases which increase the temperature of the environment and the deterioration of the ozone layer. Chemical equilibrium was used to estimate the species produced by adiabatic gasification with different air-vapor mixtures. By running the NASA CEA software (chemical equilibrium with applications), a thermochemical simulation was performed under two parameters: the equivalence ratio (ER) defined as stoichiometric air/air supplied to the reactor (1.5-6) and the steam-fuel ratio (0-1). Thus, the syngas composition showed the following ranges: CO (0% -14.7%), H2 (0% - 36.7%), CH4 (0% -4.3%), Co2 (17% -22.7%). The calorific value of the gases and the energy conversion power were also calculated with the composition of the gases. Then, The Higher Heating Value (HHV) was calculated whose values aim to conclude that syngas generated can be classified as a poor fuel gas since the HHV ranged between 1992 kJ/SATP m3-7537 kJ/SATP m3.
Keywords: Chemical balance; Gasification; Calorific value; Corn straw
Introductions
The current and growing consumption of energy and the increase of toxic NOx, SOx and greenhouse gases produced from the combustion of fossil fuels, are problems that require effective solutions that meet the energy needs of society in a sustainable manner. Fuel biomass, which includes energy crops and a wide range of municipal, agricultural and animal waste, serves as renewable resources for energy conversion processes in thermal energy conversion processes such as complete combustion and gasification. The use of biomass in the above mentioned processes does not increase the concentration of Co2 in the atmosphere because biomass is a carbon neutral fuel resource. For combustion processes, it is advisable to use biomass with high calorific value and low ash content. Biomass with low calorific value results in poor and unstable combustion whereas biomasses with high ash content cause problems in burners and boilers due to the slag and dirt produced. Complete combustion and gasification of biomass with air have been deeply studied during the last decades [1-16].
The biomass gasification is a thermal process to produce syngas which can be an alternative solution since biomass is a neutral fuel in the production of Co2 [10]. Additionally, biomass can also be used as fuel in combustion processes for power generation; however, these processes usually occur at high temperatures that favor the formation of NOx and SOx [17-19]. The use of biomass (energy crops, agricultural and municipal waste, etc.) as fuel in gasification processes for power generation not only alleviate the high demand for energy but also avoids pollution caused by fossil fuels and inadequate management of waste.
Materials & Methods
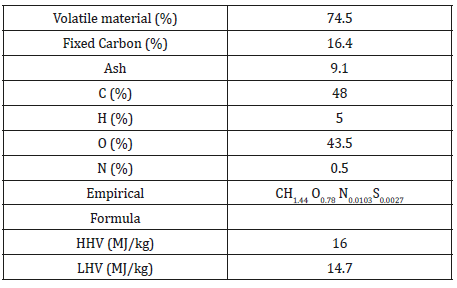
The biomass samples of corn straw were analyzed under ultimate and proximate analysis. Thus, preparation was performed under the ASTM D2013 standard for proximate analysis, which were carried out under the following standards: (ASTM D3302), (ASTM D3175/D7582), (ASTM D4239), (ASTM D3172), (ASTM 2492), (ASTM D5865). The elemental analysis of carbon, hydrogen, and nitrogen (ASTM D5373) as follows: (ASTM 2492), (ASTM D3174-12 / D7582-15). The results of these analyses are shown in Table 1.
Table 1:Proximate and Ultimate analysis on a dry basis.
Then the software CEA (Chemical equilibrium and applications) provided by NASA was used to estimate the adiabatic composition of the species produced (about 150 species). This software makes use of chemical equilibrium libraries to calculate the molar fractions of the species generated by the process.
Results & Discussion
The empirical equation and the enthalpy of biomass formation can be determined using mass conservation and proximate and ultimate analysis. The results and the simulation parameters are presented in Table 1 and Table 2 respectively.
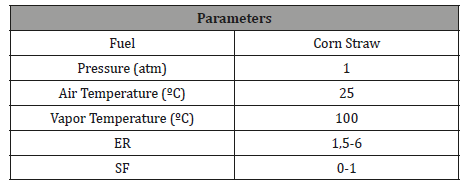
Table 2:Operating conditions for the model.
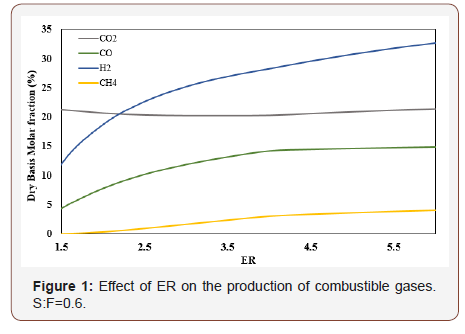
The dry basis molar fraction vs. ER of the species produced and studied during the process is presented in Figure 1.
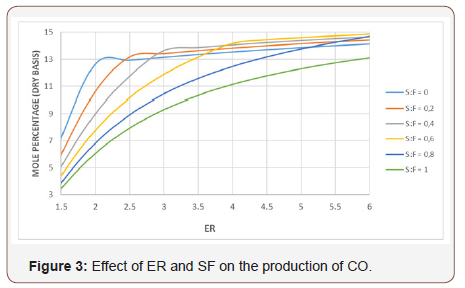
Figures 2 & 3 show the effect of ER and SF in the production of Co2 and CO respectively. The Co2 curves show a similar behavior for any SF value, but for ER values range (1-3), Co2 concentration tends to decrease and for ER between 3-6, the production of Co2 increases. The CO curves keep a similar shape for any SF, but for ER values from 1.5 to 2.5 they have a higher slope than that of those curves for ER values ranging (2.5-6).

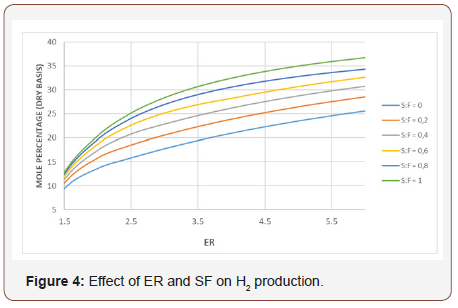
The inflection points in Figure 2 indicate that for any ER points the effect of temperature on Co2 production begins to be more important than the CO yield. The reaction C + o2 → Co2 is favored at low temperatures while the reaction C + 1/2 o2 → CO is important at high temperatures [10]. Figure 4 shows the effect of ER and SF on the production of H2. Increasing the ER for constant SF values, means more H atoms available for the C vapor reactions. As a result of this, H2 production is increased while CO production tends to decrease especially at high ER. By increasing the SF maintaining the ER constant, the production of Co2 and H2 increases but the production of CO decreases. This is due to the fact that by increasing SF the gasification process occurs in an environment rich in H2O that favors the reactions that produce mixtures rich in H2 and Co2. It can be observed that the production of H2 is more sensitive to changes of ER than that of SF. The calorific value of the gases is presented in Table 3 as a function of the ER and SF. As the ER increases, the HHV of the gases increases because of the increase of the production of H2, CO, and CH4. The gases produced at high SF are rich in H2 which makes them very attractive from the environmental point of view since the combustion of H2 produces heat and H2O, i.e at SF= 0.8 and an ER=6, it is found the highest calorific value obtained in the gasification process.
Table 3:The calorific value of the combustible gases generated (KJ/ m^3).
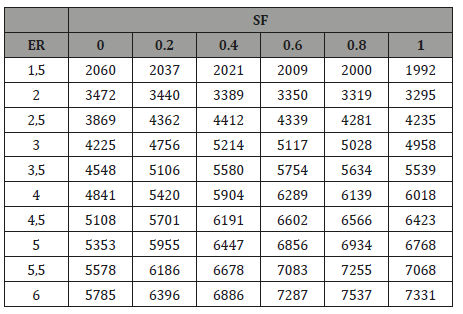
High values of ER (ER> 4) imply low oxygen supplied to the reactor therefore there is not enough oxygen for the combustion of fixed carbon (FC) and pure carbon begins to appear as a byproduct. In other words, at ER> 4 the process tends to be pyrolysis that produces carbon, gases, and ash.
In general, mixtures rich in H2 and CH4 have a better conversion efficiency due to their high calorific values, compared with that of CO.
Conclusion
• To obtain mixtures of gases rich in hydrogen it is necessary to carry out the process in an environment rich in water vapor.
• The best conditions to obtain mixtures rich in hydrogen are with high values of the ER.
• In general, the production of different combustible gases is more sensitive to variations in the ER than to variations in the SF.
• To obtain a maximum calorific value and a higher HHV of corn straw, it is important to work with an ER 6 and an SF between 0.6 and 1.
Acknowledgement
The authors of the current paper would like to acknowledge for the financial supports to Universidad ECCI and especially the Vice- Chancellery for Research Emeterio Cruz.
Conflict of Interests
This manuscript has not been published and is not under consideration for publication elsewhere. Moreover, we declare having no conflicts of interest to disclose.
References
- G Gordillo, K Annamalai (2010) Adiabatic fixed bed gasification of dairy biomass with air and steam. Fuel 89(2): 384-391.
- J Bonilla, G Gordillo (2017) Adiabatic Fixed-Bed Gasification of Colombian Coffee Husk Using Air-Steam Blends for Partial Oxidation. J Combust 2017: 1-26.
- GM Joselin Herbert, A Unni Krishnan (2016) Quantifying environmental performance of biomass energy. Renew Sustain Energy Rev 59(C): 292- 308.
- H Li, X Zhang, L Liu, S Wang, G Zhang (2017) Proposal and research on a combined heating and power system integrating biomass partial gasification with ground source heat pump. Energy Convers Manag 145: 158-168.
- L Liu, Y Huang, J Cao, C Liu, L Dong, et al. (2018) Experimental study of biomass gasification with oxygen-enriched air in fluidized bed gasifier. Sci Total Environ 626: 423-433.
- P Rao, M Muller (2007) Industrial Oxygen : Its Generation and Use Description of Technologies. Pp. 124-135.
- SK Sansaniwal, K Pal, MA Rosen, SK Tyagi (2017) Recent advances in the development of biomass gasification technology: A comprehensive review. Renew Sustain Energy Rev 72: 363-384.
- TR Pacioni, D Soares, M Di Domenico, MF Rosa, R De FP Muniz Moreira, et al. (2016) Bio-syngas production from agro-industrial biomass residues by steam gasification. Waste Manag 58: 221-229.
- X Chen (2016) Economic potential of biomass supply from crop residues in China. Appl Energy 166: 141-149.
- SK Sansaniwal, MA Rosen, SK Tyagi (2017) Global challenges in the sustainable development of biomass gasification: An overview. Renew Sustain Energy Rev 80: 23-43.
- R Junga, S Werle (2016) Experimental Analysis of the Fixed Bed Gasification Process of. Renew Energy no September, pp. 14-16.
- FM Gírio, C Fonseca, F Carvalheiro, LC Duarte, S Marques, et al. (2010) Hemicelluloses for fuel ethanol: A review. Bioresour Technol 101(13): 4775-4800.
- YA Lenis, JF Pérez, A Melgar (2016) Fixed bed gasification of Jacaranda Copaia wood: Effect of packing factor and oxygen enriched air. Ind Crops Prod 84: 166-175.
- Y Yang, JG Brammer, ASN Mahmood, A Hornung (2014) Intermediate pyrolysis of biomass energy pellets for producing sustainable liquid, gaseous and solid fuels. Bioresour Technol 169: 794-799.
- Z Zhang, S Pang (2017) Experimental investigation of biomass devolatilization in steam gasification in a dual fluidised bed gasifier. Fuel 188: 628-635.
- L Wilson, GR John, CF Mhilu, W Yang, W Blasiak (2010) Coffee husks gasification using high temperature air/steam agent. Fuel Process Technol 91(10): 1330-1337.
- N Cerone, F Zimbardi, A Villone, N Strjiugas, EG Kiyikci (2016) Gasification of Wood and Torrefied Wood with Air, Oxygen, and Steam in a Fixed-Bed Pilot Plant. Energy and Fuels 30(5): 4034-4043.
- WH Chen, BJ Lin, MY Huang, JS Chang (2014) Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour Technol 184: 314-327.
- P Basu (2013) Biomass Gasification, Pyrolysis and Torrefaction, First edn. © 2010 Elsevier Inc.
-
Galindo José, Gamba Iván, Amaris María, Bonilla Javier. Simulation of Adiabatic Gasification of Corn Straw using Air-Steam Blends as an Oxidizing Agent. Glob J Eng Sci. 1(3): 2019. GJES.MS.ID.000515.
-
Chemical balance, Gasification, Calorific value, Corn straw, Temperature, Water, Calorific value, Chemical equilibrium, Biomass, Energy crops, Agricultural, Municipal waste
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






