 Short Communication
Short Communication
Could Abnormal Distribution of Interstitial Cells of Cajal be Involved in Gastrointestinal Disorders in Patients with Parkinson’s Disease?
HOU Miaomiao1, MAO Xiaowei1, LIU Yanqun, HOU Xiaojun* and BI Xiaoying
1HOU miaomiao and MAO xiaowei contributed equally to this article, china
HOU Xiaojun, Department of Neurology, Changhai Hospital attached to The Second Military Medical University, 168 Changhai Road, Shanghai, China.
Received Date: November 10, 2018; Published Date: November 27, 2018
Abstract
Objective: To study the role of interstitial cells of Cajal (ICC) in the pathogenesis of gastro-intestinal motility disorders in Parkinson’s disease (PD).
Methods: The distribution of ICCs was studied by immunohistochemical analysis using anti c-kit antibodies in 4 cases of PD patients and 4 paired non-PD patients.
Results: ICCs were located in the inner part of the circular muscle and around the myenteric plexus between the two muscle layers. The number of c-kit+ ICCs was remarkably reduced or even disappeared in patients with PD.
Conclusion: The lack of ICCs may lead to the abnormal spontaneous electrical activity and conduction which gives rise to gastrointestinal motility disorders in PD.
Keywords: Parkinson’s disease; Gastro-intestinal motility disorders; Interstitial cells of Cajal
Introduction
Parkinson’s disease (PD) is a common degenerative disease of the nervous system in elderly people. The main clinical manifestations are tremor and rigidity. In addition to the typical motor symptoms, PD patients also have a higher incidence of gastrointestinal disorders, of which constipation is the most common, with a foreign incidence of 67% [1], the incidence in domestic of more than 50% [2]. Interstitial cells of Cajal (ICC) plays an important role in gastrointestinal motility as a pacemaker and transduction agent of gastrointestinal rhythmic electrical activity. To date, studies on the pathogenesis of PD gastrointestinal disorders have not involved the ICC pathway. In this study, we used anti-C-kit immunohistochemistry to observe the distribution of ICCs in the intestinal tract of PD patients, so as to explore the role of ICCs in the gastrointestinal dysfunction of PD.
Our research was approved by the ethics committee of Shanhai Changhai Hospital with No. CHEC2018-081. From December 2009 to May 2017, 4 cases of PD inpatients and 4 paired cases of non- PD inpatients were operated due to gastrointestinal tumor. We took the end close to the normal tissue and did an immunohistochemical analysis using anti c-kit antibodies (Table 1).
Table1: PD patients’ characteristics.
The colon showed two c-kit positive cells: one in the mucosal layer with a large cell body and a round shape that was identified as mast cells by toluidine blue staining. Another located in the circular muscle layer and around the myenteric plexus between the circular and longitudinal muscle layers, spindle-shaped, along its longitudinal axis having two or three long protrusions, larger nuclei, visible in the cytoplasm of brown particulate matters, was identified as ICCs.
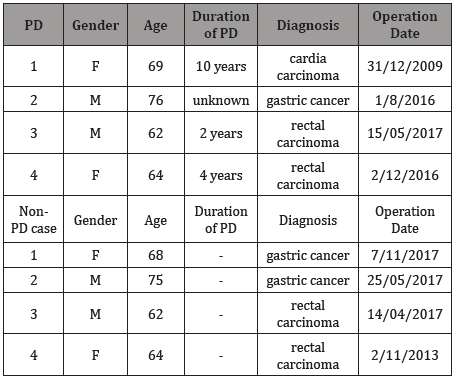
In the control case 1: ICCs are mainly distributed on the inner surface of the circular muscle, cling to the smooth muscle cells, and run parallel to the smooth muscle (Figure 1). Around the nerve plexus between the circular and longitudinal muscle layers also distributed a large number of ICCs, with obvious boundaries with the surrounding tissue (Figure 1 & 2).

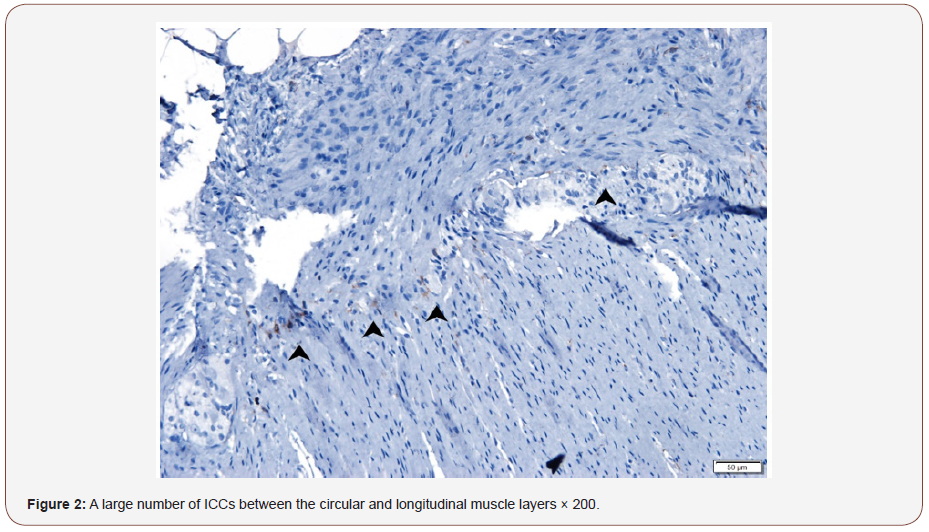
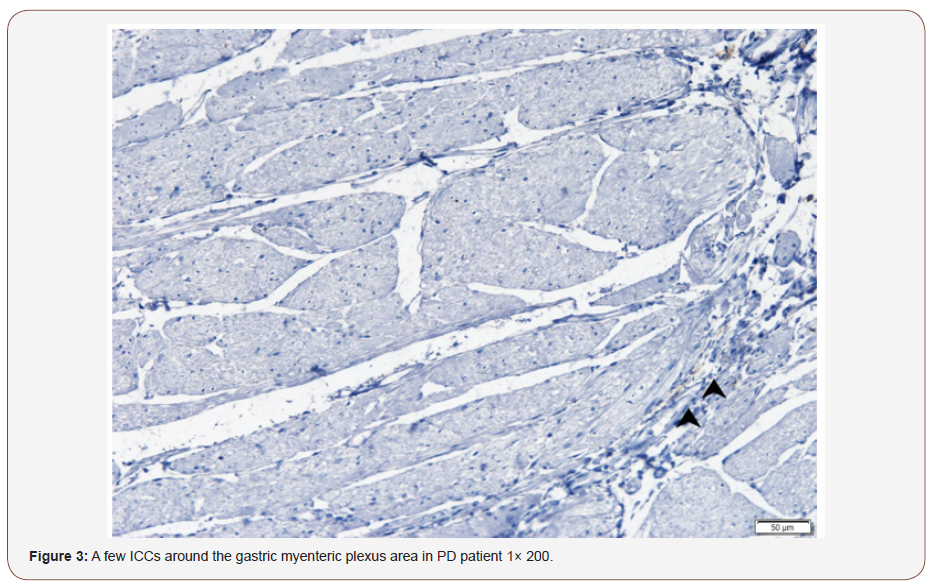
In the PD patient 1 and 2: ICCs in the circular muscle layer were significantly reduced, or even absent. Around the myenteric plexus region distributed only a few ICCs. ICCs are far away from each other. The connection is not obvious (Figure 3& 4).
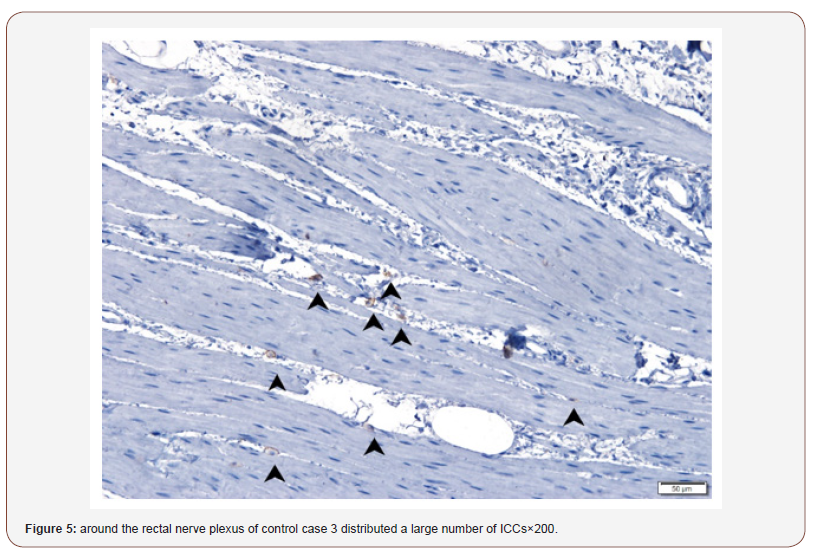
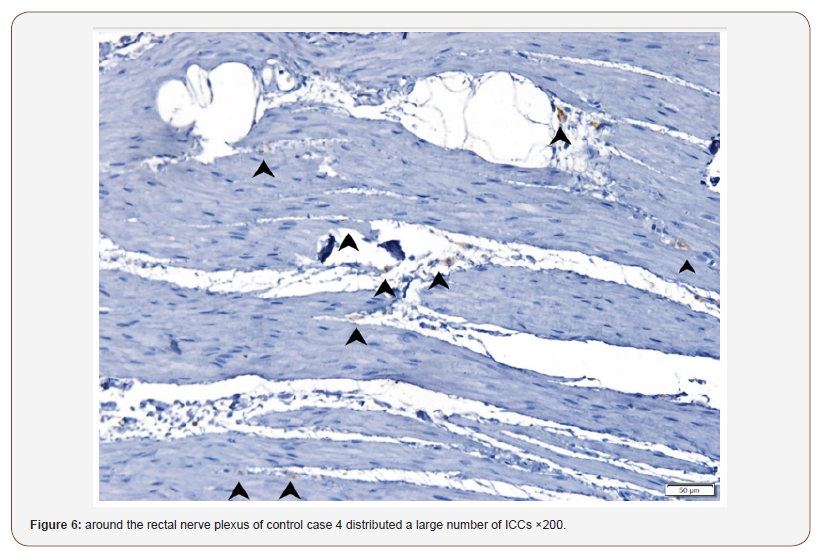
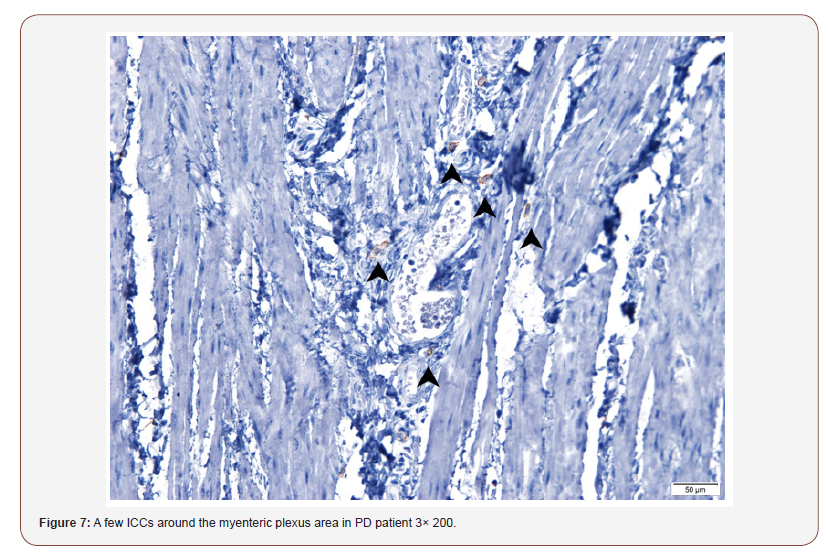
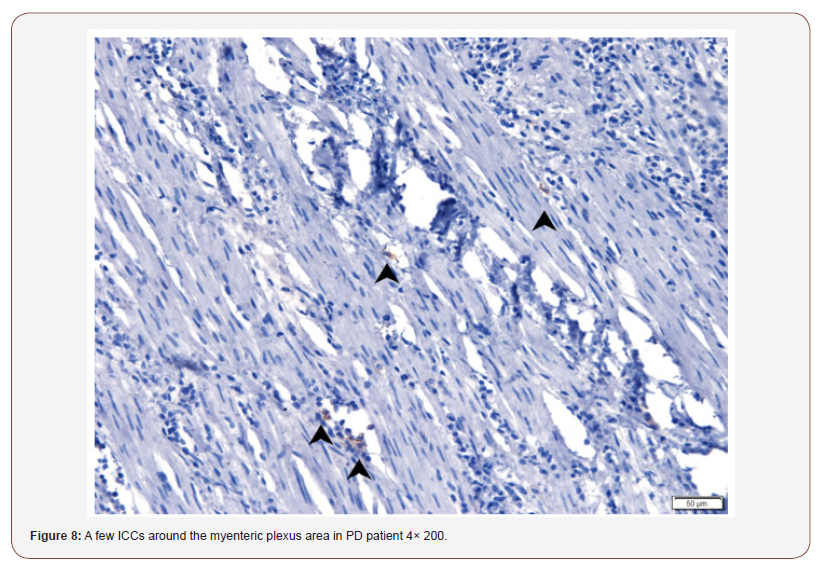
In the control case 3 and 4: Around the rectal nerve plexus distributed a large number of ICCs (Figure 5 & 6), while in the PD patient 3 and 4, ICCs were significantly reduced (Figure 7 & 8)
Discussion
Gastrointestinal ICCs have three main functions: the first is to participate in electrical activity transmission as a pacemaker of rhythmic electrical activity; the second is as a mediator between neural impulse transmission and smooth muscle contraction; the third is to regulate neurotransmitters. The slow waves from ICCs propagate through the gap junction to smooth muscle cells, activate smooth muscle cells and produce action potentials that cause the smooth muscle to contract [3]. ICCs-related diseases include achalasia, hypertrophic pyloric stenosis, chronic pseudo-intestinal obstruction, Hirschsprung disease, inflammatory bowel disease, slow transit constipation, diabetic gastroparesis, Oddi sphincter dysfunction and other gastric Intestinal motility disorders and gastrointestinal stromal tumors [4].
PD is a common neurovegetative disease in the elderly with tremor, rigidity, akinesia and postural instability and gait disorders as the main clinical symptoms. In recent years, gastrointestinal symptoms that plague PD patients such as dysphagia, delayed gastric emptying, constipation are gaining more and more attention. Many factors may affect the gastrointestinal function, such as the use of anti-Parkinson drugs, long-term use of laxatives, etc., and PD disease itself may also be one of the main reasons [5]. Singaram et al. [6] found that the number of dopaminergic neurons in the intestinal muscle layer in the colon of patients with PD was significantly reduced than that in the normal controls and idiopathic constipation group. This result suggests that PD patients may have peripheral dopamine depletion. The intestinal dopaminergic neurons are just a few of the many neuronal populations in the enteric nervous system. Non-dopaminergic neurons in the gut, such as nitric oxidergic neurons, cholinergic neurons may also be involved in the disease process [7,8]. However, it is unclear which mechanism of PD causes changes of these gastrointestinal neurons and how they affect gastrointestinal activities. To date, studies on the pathogenesis of PD gastrointestinal disorders have not involved the ICCs pathway. The results of this study show that the number of intestinal ICCs in patients with PD significantly reduced or even disappeared, compared with the normal bowel. Slow wave is considered as the starting potential of smooth muscle, controlling the rhythm of smooth muscle contraction and determining the direction, rhythm and speed of peristalsis. The reduction of ICCs will inevitably weaken the stimulating effect on the slow wave of intestine smooth muscle. In addition, as the intestinal nerve, ICCs and smooth muscle cells form the basic functional units that deliver mechanical effects, the lack of ICCs may lead to the interruption of this chain, and probably the blockage of the neurotransmission. Therefore, the lack of intestinal ICCs in PD patients eventually leads to autonomous bowel movement disorder. It is not yet clear whether the reduction of ICCs is a primary risk factor or a secondary pathological change. However, it is certain that lack of ICCs in pathological bowel is one of the pathological changes of PD, just as the depletion of dopaminergic neurons.
There are some shortcomings in this study: First, the sample size is relatively small, and we did not get a significant difference statistically. Because ICCs mainly exist in the gastrointestinal muscular layers, specimens must be surgically resected but not endoscopically. Second, most of the resected samples came from tumor patients. Although the samples taken close to the normal tissue end, we cannot exclude the tumor-induced destruction.
Conclusion
With our extremely small number of PD tact specimen, we found that ICCs were located in the inner part of the circular muscle and around the myenteric plexus between the two muscle layers. The number of c-kit+ ICCs was remarkably reduced or even disappeared in patients with PD. The lack of ICCs may lead to the abnormal spontaneous electrical activity and conduction which gives rise to gastro-intestinal motility disorders in PD. In the future, it is hoped that the study of ICCs by PD animal model may provide new ideas for revealing the mechanism of gastrointestinal dysfunction in PD.
Acknowledgemnet
We thank Dr. Zheng Jianming and other doctors from the department of pathology for their support to our study.
Conflict of Interest
I declare there are no relevant conflicts of interest.
References
- Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, et al. (2012) Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol 72: 893-901.
- Hasegawa K (2012) Does early detection of non-motor symptoms facilitate early treatment of Parkinson’s disease? Brain Nerve 64: 453- 461.
- Mathers SE, Kempster PA, Swash M, Lees AJ (1988) Constipation and paradoxical puborectalis contraction in anismus and Parkinson’s disease: a dystonic phenomenon? J Neurol Neurosurg Psychiatry 51: 1503-1507.
- Rolle U, Piaseczna-Piotrowska A, Puri P (2007) Interstitial cells of Cajal in the normal gut and in intestinal motility disorders of childhood. Pediatr Surg Int 23: 1139-1152.
- Mitsui R, Komuro T (2002) Irect and indirect innervation of smooth muscle cells of rat stomach, with special reference to the interstitial cells of Cajal. Cell Tissue Res 309: 219-227.
- Singaram C, Ashraf W, Gaumnitz EA (1995) Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 346(8979): 861-864.
- Chaumette T, Lebouvier T, Aubert P, Lardeux B, Qin C, et al. (2009) Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental Parkinsonism. Neurogastroenterol Motil 21(2): 215-222.
- Anderson G, Noorian AR, Taylor G, Anitha M, Bernhard D, et al. (2007) Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp Neurol 207: 4-12.
-
NHOU Miaomiao, MAO Xiaowei, LIU Yanqun, HOU Xiaojun, BI Xiaoying. Could Abnormal Distribution of Interstitial Cells of Cajal be Involved in Gastrointestinal Disorders in Patients with Parkinson’s Disease?. Arch Neurol & Neurosci. 1(5): 2018. ANN.MS.ID.000525.
-
OcParkinson’s disease, Gastro-intestinal motility disorders, Interstitial cells of Cajal, Immunohistochemical, Anti c-kit antibodies, Non-PD patients, PD patients, Circular muscle, Gastro-intestinal, Gastrointestinal motility
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






