 Research Article
Research Article
Benign Anisocoria is related to Gender
William D. Poynter*
Department of Psychology, Western Carolina University; Cullowhee, NC, USA (retired)
William D. Poynter, Department of Psychology, Western Carolina University; Cullowhee, NC, USA (retired); Email: wdpoynter@icloud.com
Received Date: March 27, 2019; Published Date: April 26, 2019
Abstract
Benign or physiologic anisocoria (BA) refers to a small difference in the size of the two pupils, which is likely caused by asymmetry of sympathetic nervous system (SNS) innervation. Here I measure BA in a large sample of young adults and find that its distribution varies with gender. Females presented a small but significant bias to the right (right pupil larger than left), whereas males exhibited a larger and significant bias to the left (left pupil larger than the right). The sex difference was primarily attributable to right-pupil size, which was significantly larger for females than males (no significant difference in left-pupil size). Based on the premise that BA is caused by SNS asymmetry, these results suggest that SNS lateralization is more common in males than females and biased to the opposite side of the brain. Future studies that measure bilateral brain activity in addition to pupil size and other measures of SNS function (e.g., electrodermal response) are needed to validate the relationship between BA and SNS lateralization.
Keywords: Pupil size; Asymmetry; Lateralization; Autonomic function; Anisocoria; Gender differences
Introduction
Pupil-size asymmetry can be a sign of serious medical pathology, but small differences are common in healthy individuals. This condition is called benign or physiologic anisocoria (BA) and has been described in numerous ophthalmology publications [1-6].
[7] presents compelling evidence that this condition is caused by sympathetic nervous system asymmetry, one eye receiving greater SNS activation than the other. Consistent with this thesis, asymmetry has been observed in several functional domains of the SNS, and most results suggest that the right side of the brain is more sympathetically active than the left. For example, stimulating the right-hemisphere with left-eye monocular viewing produces larger pupil dilation than when the left-hemisphere is stimulated [8,9]. Right-hemisphere lateralization has also been found in sympathetic cardiovascular control [10-15]. Likewise, many studies provide evidence that the right-hemisphere plays a special role in sympathetically-driven emotional experience [16-21].
While these studies present convincing evidence of SNS lateralization, the distribution of this asymmetry has not been quantified (owing in part to the relatively small sample sizes in these studies). Do most individuals exhibit right- lateralized SNS function? Are there sex differences in the direction and magnitude of SNS laterality, as there are in other domains of psychological function and brain structure? [22]
The distribution of BA may provide information to help answer these questions. BA is typically measured by subtracting the diameter of the left pupil from that of the right, so its sign indicates which pupil is larger, and thus which side of the brain may be more active with regard to SNS function. If most individuals do in fact exhibit right- lateralized SNS function, then the central tendency of the BA distribution should reflect this laterality and be shifted or skewed away from a value of 0.0. Likewise, if gender, ethnicity, and other participant variables relate to the incidence and direction of SNS asymmetry, then these factors should also influence the BA distribution. One of the advantages of using BA as a marker of SNS asymmetry is that pupil size can be easily and unobtrusively measured in parallel with other physiologic and psychological variables, thus providing a new approach to studying the relationship between autonomic and psychological function.
In this report I present statistical analysis of two sets of BA data, and find evidence of laterality in both sets (i.e., non-zero Mean BA). The first set came from a psychology experiment [27] in which pupillometry data were collected while subjects engaged in tasks that varied in cognitive/attentional load and illumination levels. Thus, BA levels in that study were likely influenced by variations in light-evoked and task-evoked pupillary responses [28]. The second set was measured on a archive of facial photographs taken while subjects were at rest, with no cognitive demands, similar in that respect to ophthalmology studies cited earlier [1-6]. Analysis of these data replicate our earlier findings of 1) substantial individual differences in BA (and presumably SNS asymmetry), 2) a small but significant bias in the direction of lateralization (right pupil smaller than left), and 3) a gender difference in the direction and magnitude of Mean BA, suggesting a similar gender difference in SNS asymmetry.
Methods
A total of 397 high-resolution (3008 X 2000) color face photographs were obtained from the Chicago Face Database collection, which can be obtained at:
http://faculty.chicagobooth.edu/bernd.wittenbrink/cfd/ download/download.html
[14] describes the purpose of the database, photographic methods, face-feature measurements, etc. Photographs were of relatively young individuals (Mean age = 29.6) posed in wellstandardized front-facing posture and neutral facial expression (see Figure 1). The subjects were of 4 ethnic classes (Asian (F = 51, M = 50); Black (F = 48, M = 46); Latino (F = 48, M = 50); White (F = 52, M = 53)). I used a photo-analysis software application (OMAX Toup View) to visually outline the pupil contour and thus measure pupil diameter (Figure 1b). Two measurements were taken on each pupil and the average diameter used in further analysis.
Results
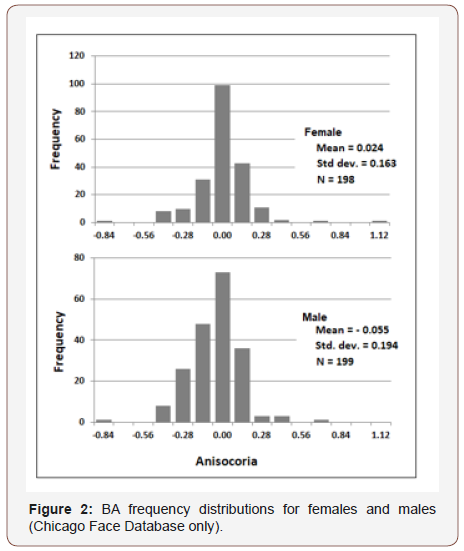
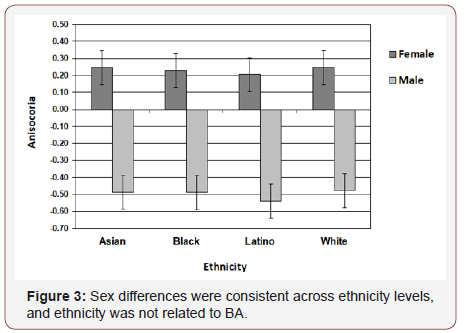
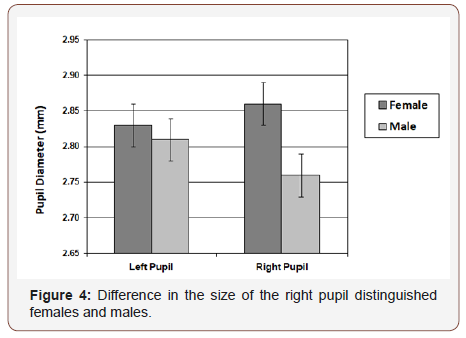
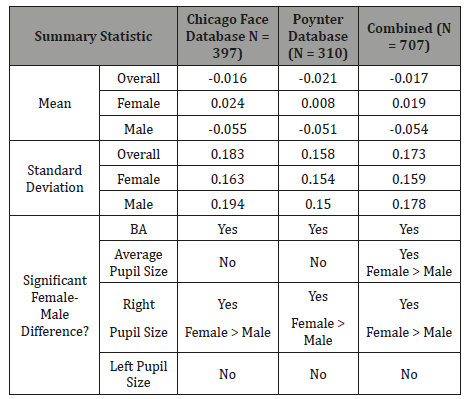
Chicago Face Database. The difference in diameter of right and left pupil (in millimeters) served as the measure of BA (R - L). Figure 2 presents frequency distributions for females and males. Mean values were significantly different from zero (females: t (197) = 2.3, p. = 0.02; males: t (198) = 4.1, p. <0.001), and also significantly different from each other (t (395) = 4.95, p <0.001). 30% of females exhibited a positive BA bias and 23% a negative bias; 42% of males exhibited a negative BA bias and 23% a positive bias. Gender differences were consistently observed across ethnicity levels, but ethnicity was not related to BA (Figure 3). Figure 4 shows that the gender difference was primarily driven by right-pupil size, which was significantly different between males and females (F = 2.85; M = 2.75: t (395) = 2.26, p. = 0.02). The difference in left pupil size was not significant.
Similar gender differences were observed in [27], but a different BA metric was used there. So, for purpose of comparison, Table 1 presents summary statistics for both studies using the (R-L) metric. Despite substantial demographic differences in the subject samples and context within which subjects were measured (e.g., age and ethnicity mix, illumination levels, subject’s visual/mental activities during measurement), results were quite similar. Data from both studies were combined to provide an overall summary (Table 1 & Figure 5).
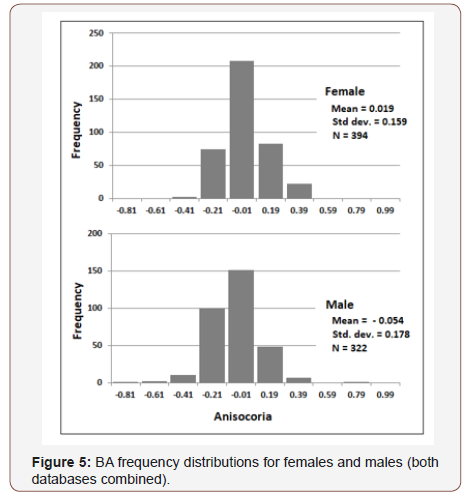
Table 1: BA summary statistics.
Discussion
Benign anisocoria was measured in two studies (total N = 707). The data were broadly distributed (Figure 2&5), and Mean BA for both data sets was slightly negative, as found in previous ophthalmology studies (LoewenFeld (1977): BA = ~ - 0.03, N = 850; Smith, et al. (1979): BA = - 0.06, N=150). So, if BA is in fact a marker of SNS asymmetry, these data suggest SNS activation is on average (across genders) greater for the left pupil than for the right. And since right- hemisphere dominance has been reported in a number of SNS-lateralization studies [8,10,12,14,15,29], greater left-pupil SNS activation paired with right-hemisphere SNS dominance may suggest that the SNS pathways between cortex and pupil are contralateral (i.e., right brain activates left pupil). But more importantly, the present study found that the distribution of BA is shifted to the left of 0.0 for males (left pupil larger than right) and to the right of 0.0 for females (left pupil smaller than right). Moreover, Table 1 shows that the absolute difference of Mean BA from 0.0 was larger for males than females. Thus, SNS function may be more asymmetric for males than females and lateralized to the opposite side of the brain. This speculation is consistent with an extensive body of research indicating that the male brain is less symmetric than the female brain, both in terms of function and architecture [21-23,25,26].
Conclusion
This study helps to clarify the statistical distribution of benign anisocoria in the human population and may provide new information about the distribution of SNS asymmetry. The gender differences in BA suggest similar differences in SNS lateralization, which may in turn relate to other physiologic, behavioral, and cognitive traits (e.g., personality and attentional function [27]). Future studies that directly measure bilateral brain activity in addition to pupil size and other measures of SNS function (e.g., electrodermal response) are needed in order to validate the relationship between BA and SNS lateralization. Assuming this relationship is confirmed, BA may have value as a convenient, inexpensive, and unobtrusive measure of SNS asymmetry.
Acknowledgment
None.
Conflicts of Interest
No Conflicts of interest.
References
- Bremner FD, Booth A, Smith SE (2004) Benign alternating anisocoria. Neuro-Ophthalmology 28(4): 129-135.
- Ettinger ER, Wyatt HJ, London R (1991) Anisocoria: Variation and clinical observation with different conditions of illumination and accommodation. Investigative Ophthalmology & Visual Science 32(3): 501-509.
- Lam BL, Thompson HS, Corbett JJ (1987) The prevalence of simple anisocoria. American Journal of Ophthalmology104: 69-73.
- Lam BL, Thompson HS, Walls RC (1996) Effect of light on the prevalence of simple anisocoria. Ophthalmology 103(5): 790-793.
- Loewenfeld IE (1977) “Simple, central” anisocoria. A common condition seldom recognized. Transactions of the American Academy of Ophthalmology and Otolaryngology 83(5): 832-839.
- Smith SA, Ellis CJK, Smith SE (1979) Inequality of the direct and consensual light reflexes in normal subjects. British Journal of Ophthalmology 63: 523-527.
- Rosenberg ML (2008) Physiologic Anisocoria: A manifestation of a physiologic sympathetic asymmetry. Neuro-Ophthalmology 32: 147- 149.
- Burtis DB, Heilman KM, Mo J, Wang C, Lewis GF, et al. (2014) The effects of constrained left versus right monocular viewing on the autonomic nervous system. Biological Psychology 100: 79-85.
- Wang C, Burtis DB, Ding M, Mo J, Williamson JB, et al. (2017) The effects of left and right monocular viewing on hemispheric activation. Journal of Clinical and Experimental Neuropsychology 40(2): 198-204.
- Hilz MJ, Dutsch M, Perrine K, Nelson PK, Rauhut U, et al. (2001) Hemispheric influence on autonomic modulation and baroreflex sensitivity. Annals of Neurology 49(5): 575-584.
- Mc Ginley JJ, Friedman BH (2015) Autonomic responses to lateralized cold pressor and facial cooling tasks. Psychophysiology 52(3): 416-424.
- Mc Ginley JJ, Friedman BH (2015) Autonomic responses to lateralized cold pressor and facial cooling tasks. Psychophysiology 52(3): 416-424.
- Oppenheimer SM, Gelb A, Girvin JP, Hachinski, VC (1996) Cardiovascular effects of human insular cortex stimulation. Neurology 42: 1727-1732.
- Oppenheimer SM, Kedem G, Martin WM (1996) Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clinical Autonomic Research 6: 131-140.
- Tegeler CH, Shaltout HA, Tegeler CL, Gerdes L, Lee SW (2015) Rightward dominance in temporal high-frequency asymmetry corresponds to higher resting heart rate and lower baroreflex sensitivity in a heterogeneous population. Brain and Behavior 5(6): e00343.
- Zamrini EY, Meador KJ, Loring DW, Nichols FT, Lee GP (1990) Unilateral cerebral inactivation produces differential left/right heart rate responses. Neurology 40(9): 1408-1411.
- Canli T, Desmond JE, Zhao Z, Glover G, Gabrieli JDE (1998). Hemispheric asymmetry for emotional stimuli detected with fMRI. Neuroreport 9(14): 3233–3239.
- Davidson RJ (1993) Parsing affective space: perspectives from neuropsychology and psychophysiology. Neuropsychology 7(4) 464– 475.
- Demaree HA, Everhart DE, Youngstrom EA, Harrison DW (2005) Brain Lateralization of Emotional Processing: Historical Roots and a Future Incorporating “Dominance”. Behavioral and Cognitive Neuroscience Reviews 4(1): 3-20.
- Sackeim HA, Gur RC, Saucy MC (1978) Emotions are expressed more intensely on the left side on the face. Science 202: 433-435.
- Silberman EK, Weingartner H (1986) Hemispheric lateralization of functions related to emotion. Brain and Cognition 5: 322-353.
- Williamson JB, Harrison DW (2003) Functional cerebral asymmetry in hostility: A dual task approach with fluency and cardiovascular regulation. Brain & Cognition 52: 167-174.
- Cahill L (2006) Why sex matters for neuroscience. Nature Reviews Neuroscience 7: 477-484.
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, et al. (2014) Sex Differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences 111(2): 823-828.
- Kovalev VA, Kruggel F, Von Cramon DY (2003) Gender and age effects in structural brain asymmetry as measured by MRI texture analysis. Neuroimage 19(3): 895–905.
- Mc Glone J (1980) Sex differences in human brain asymmetry: A critical survey. Behavioral Brain Science 3(2): 215-263.
- Voyer D (1996) On the magnitude of laterality effects and sex differences in functional lateralities. Laterality, 1(1): 51-83.
- Poynter WD (2017) Pupil-size asymmetry is a physiologic trait related to gender, attentional function, and personality. Laterality: Asymmetries of Body, Brain and Cognition 22(6): 654-670.
- Beatty J (1982) Task-Evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin 91(2): 276- 292.
- Ma DS, Correll J, Witten brink B (2015) Behav Res 47: 1122–1135.
-
William D Poynter. Benign Anisocoria is related to Gender. W J Opthalmol & Vision Res. 1(5): 2019. WJOVR.MS.ID.000523.
-
Pupil Size, Asymmetry, Lateralization, Autonomic Function, Anisocoria, Sex Differences, Race, Vision sciences, Ophthalmology, Optometry, Visual science.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






