 Research Article
Research Article
The Effect of Primary Surgical Technique for Treatment of Endometrial Cancer and Timing of Adjuvant Radiation Therapy
Melissa Schwartz1, Shannon Tomita1*, Valentin Kolev1, Samantha Raymond2, Jessica Overbey2, Vishal Gupta3, Stephanie V Blank1 and Manjeet Chadha3
1Department of Obstetrics, Gynecology and Reproductive Sciences, Division of Gynecologic Oncology, Icahn School of Medicine at Mount Sinai, New York, USA
2Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, USA
3Department of Radiation Oncology, Icahn School of Medicine at Mount Sinai, New York, USA
Shannon Tomita, Department of Obstetrics, Gynecology and Reproductive Sciences, Division of Gynecologic Oncology, Icahn School of Medicine at Mount Sinai, New York NY, USA.
Received Date: October 18, 2019 Published Date: November 05, 2019
Abstract
Objective: To determine if surgical approach (open, laparoscopic, robotic) influences time to initiation of adjuvant radiation therapy in women with endometrial cancer.
Methods: The National Cancer Database was used to search for patients with stage I to III endometrial cancer who received adjuvant radiation therapy from 2010-2012. Demographic, socioeconomic and clinical information was abstracted for each patient. Time to initiation of adjuvant radiation therapy was compared between groups using univariable (unadjusted) and multivariable (adjusted for all demographic and clinical variables) Cox regression models.
Results: A total of 15,480 patients were included in our study. 47.9% of patients underwent laparotomy or an unspecified surgical approach, 38.6% underwent robotic surgery, and 13.5% had a laparoscopic surgery. The distribution of time to radiation was significantly different among these groups with hazards ratios 1.0 (reference), 1.1 [95% CI 1.07, 1.15] and 1.15 [95% CI 1.02-1.06] for open, robotic and laparoscopic surgery (p<0.0001), respectively. After adjusting for covariates, younger age and worse disease status as measured by stage and grade are associated with longer wait times to radiation.
Conclusions: Women who underwent open staging surgery for endometrial cancer experienced delays from surgery to initiation of adjuvant radiation therapy as compared to women who had minimally invasive surgery.
Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in the United States, accounting for an estimated 63,230 new cases and 11,530 deaths in 2018 [1]. Women with endometrial cancer undergo surgical staging followed by adjuvant treatment as appropriate. Adjuvant radiotherapy (RT) is commonly used for patients with early-stage high-intermediate risk disease or those with advanced disease in order to prevent locoregional recurrence. The locoregional recurrence rate for early stage endometrial cancer with risk factors is up to 26% [2,3]. For patients with advanced stage disease, despite receiving systemic chemotherapy, up to 18% of patients will develop a locoregional recurrence [4].
When feasible, minimally invasive surgery is the preferred surgical modality for EC staging and treatment [5]. Laparoscopic surgical management for EC has been shown to be a safe alternative to laparotomy. Laparoscopy as compared to open techniques results in fewer short-term complications and decreased hospital length of stay [6] with similar oncological outcomes [5,7]. A meta-analysis of the literature comparing robotic-assisted laparoscopic surgery to laparotomy for EC also revealed decreased intraoperative and postoperative complication rates, decreased postoperative morbidity, improved patient-reported outcomes, including significantly shorter return to daily activities, and equivalent survival rates [8,9]. Robotic-assisted surgery for EC has also been compared with traditional laparoscopic approach and has shown equivalent short-term outcomes [10]. The use of laparoscopy and robotic-assisted surgery for EC is increasing.
Mode of surgery influences patient recovery times and therefore may influence time from surgery to adjuvant therapy. There is evidence that time intervals between diagnosis, surgery, and initiating adjuvant treatment for patients with endometrial cancer influences outcomes [11-13]. Studies have shown that a delay between hysterectomy and adjuvant RT might portend worse outcomes. Currently, literature exploring this relationship is limited. One retrospective study showed that initiating adjuvant RT more than six weeks after surgery decreased disease-specific survival for EC [11]. Others have found that local recurrence rate was associated with time interval from surgery to RT using a cutoff of nine weeks [12,13]. Given the advent of minimally invasive surgery, we sought to evaluate the impact of surgical technique on the time interval for initiating adjuvant radiation therapy for patients with endometrial cancer.
Methods
The National Cancer Database (NCDB) is a national hospitalbased cancer registry that is a joint endeavor of the American College of Surgeons and American Cancer Society. Annually, data from over 1 million patients representing 70% of all new cancer diagnoses in the United States are reported to the NCDB. Approximately 1,500 Commission on Cancer (CoC)-accredited hospitals contribute deidentified data. Data reporting is highly standardized to the CoC Registry Manuals, the American Joint Committee on Cancer and Collaborative Stage manuals, and the International Classification of Diseases for Oncology, Third Edition (ICD-O-1) [14,15]. The Institutional Review Board at the Icahn School of Mount Sinai granted exemption status for this study.
The NCDB Participant User File was used to search for patients with stage I to III EC who underwent primary surgical treatment and for whom the surgical technique was known from 2010 to 2012. Only those patients with a known cancer diagnosis prior to surgery who also received adjuvant radiotherapy were included in the study. The types of adjuvant RT delivered were external beam radiation therapy (EBRT), brachytherapy, radioisotopes, or combined EBRT with brachytherapy boost or radioisotopes. Patients who received radiation outside of the uterus, cervix, pelvis or abdomen were excluded. Demographic and socioeconomic information was abstracted for each patient, including age, year of diagnosis, race/ ethnicity, income, insurance status, and facility location. Clinical data collected included Charlson-Deyo comorbidity score, surgical approach, time to radiation, and time hospitalized. Pathologic details recorded included International Federation of Gynecology and Obstetrics (FIGO) staging and grade of disease.
Patients were categorized into three groups according to surgical approach: robotic, laparoscopic, and open or unspecified. Patients whose surgical approach was not reported were categorized as unspecified and grouped with patients who underwent open surgery by the NCDB. Demographic and clinical characteristics were compared between the groups using one-way ANOVA or Kruskal-Wallis tests for continuous measures and Chi-square tests for categorical measures. The distribution of time to adjuvant radiation by surgical approach was estimated using the Kaplan Meier method and differences between the groups were assessed using a log-rank test. Univariable (unadjusted) and multivariable (adjusted for all demographic and clinical variables) Cox regression models were estimated to assess each factor’s association with time to adjuvant radiation therapy. All analyses were conducted using SAS version 9.4 (SAS, Cary, NC).
Results
A total of 15,480 patients diagnosed with endometrial cancer from 2010 to 2012 met inclusion criteria. Of these, 47.9% had a laparotomy or unspecified surgical approach, 38.6% underwent robotic surgery, and 13.5% had a laparoscopic surgery. Although statistically significant due to the large sample size, there were no clinically significant differences between the groups based on age, income, insurance status, facility location, and Charlson-Deyo score (Table 1). The robotic group had a higher proportion of white patients compared to both the laparoscopic and open/unspecified groups. The open/unspecified group had proportionately more stage 3 and grade 3 and 4 (undifferentiated or anaplastic) patients compared to the robotic and laparoscopic groups. As expected, the length of stay in hospital was also significantly longer for open/ unspecified patients compared to patients who underwent less invasive procedures. Notably, the distribution of year of diagnosis varied by surgical group with the percentage of patients in both the robotic and laparoscopic increasing by year versus decreasing in the open/unspecified group.
Table 1: Characteristics of endometrial cancer patients treated with radiation therapy by surgical approach.
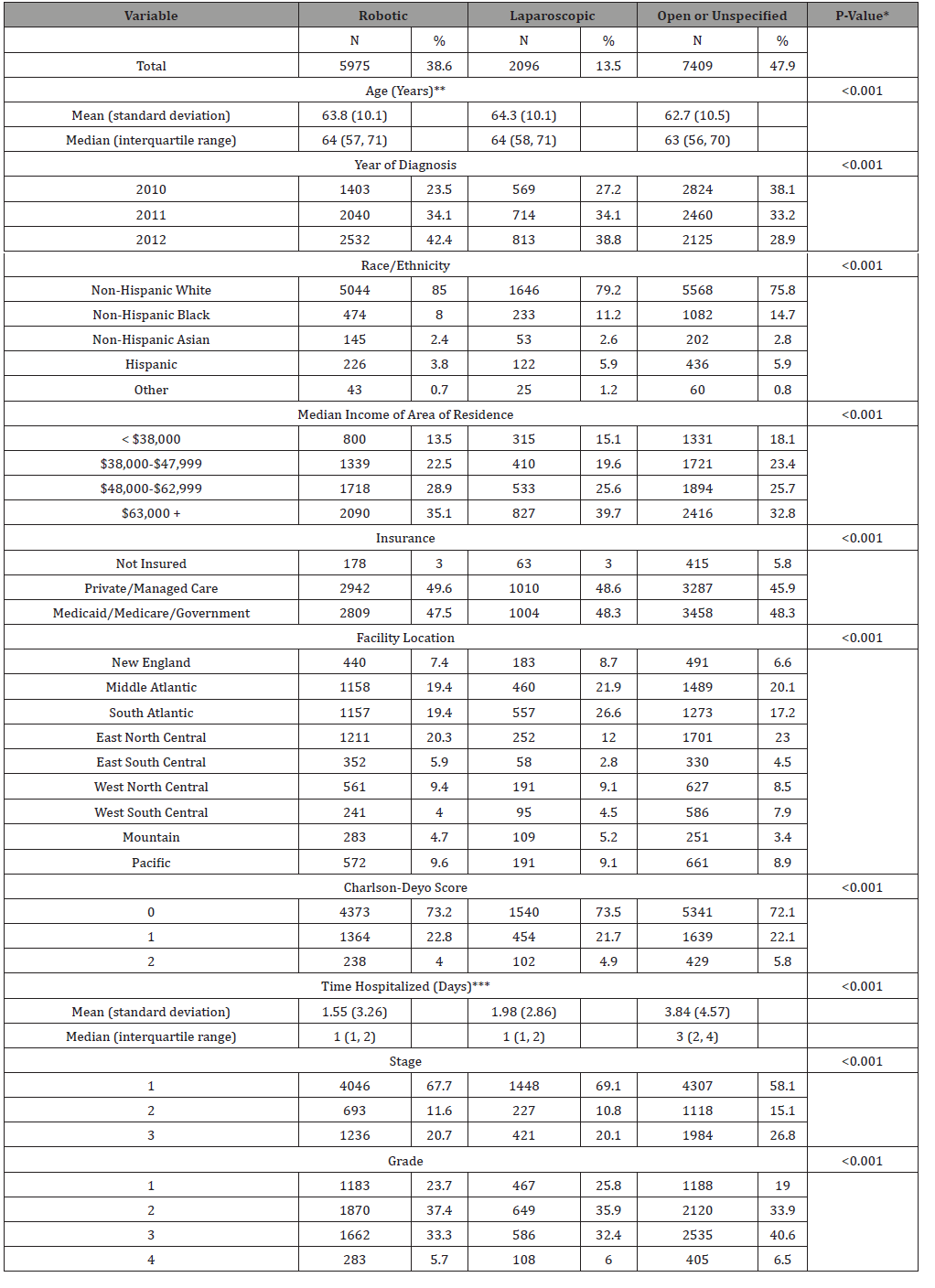
*Chi-square test unless otherwise noted
**P-value is from a one-way ANOVA
***P-value is from a Kruskal-Wallis test
Across the three surgical groups, the distribution of time to radiation was significantly different (p<0.0001, Figure 1). Although median times to radiation are similar across the groups, the 75th percentile of the open or unspecified group is 108 days compared to 98 and 92 days for the robotic and laparoscopic groups respectively, meaning that more open patients had a longer wait time to radiation compared to robotic and laparoscopic patients (Table 2).
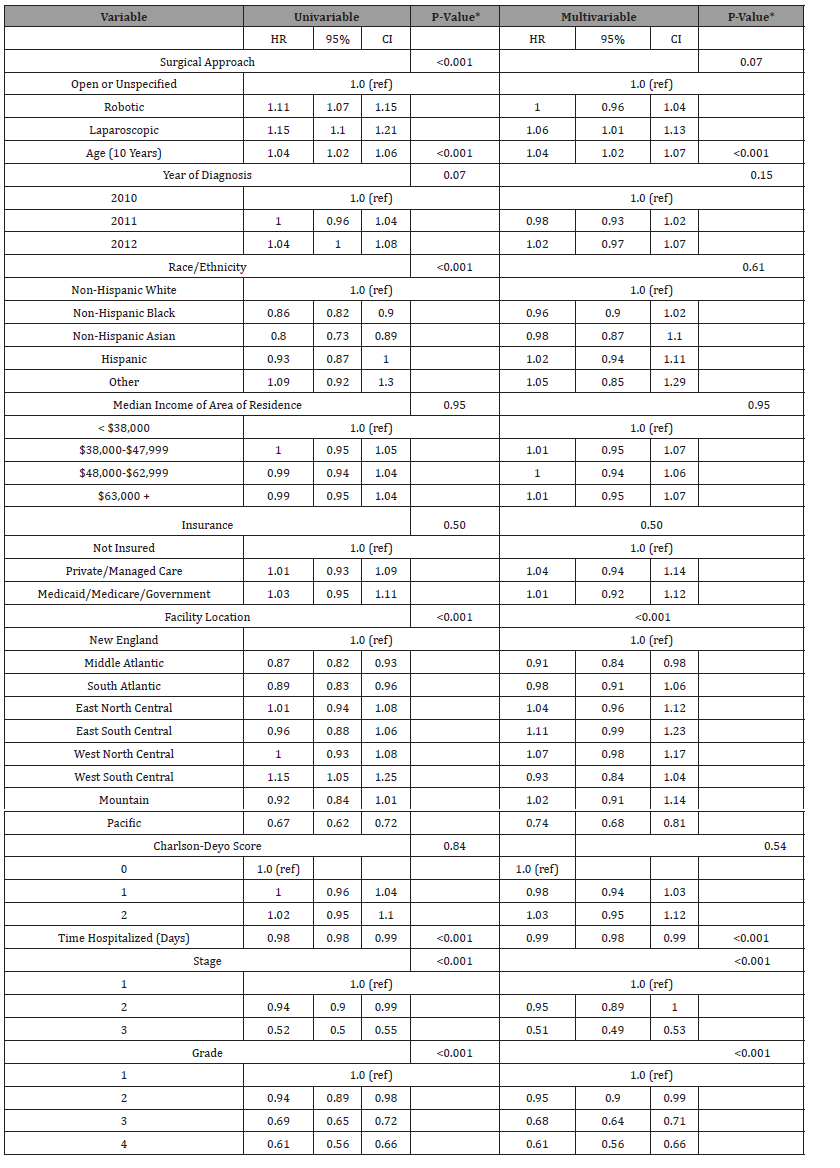
In unadjusted analyses, compared to open/unspecified patients, at any given time laparoscopic patients were 1.15 times more likely to initiate radiation therapy (95% CI 1.10,1.21) and robotic patients were 1.11 times more likely to initiate radiation therapy (95%CI 1.07, 1.15; Table 3). Age, race/ethnicity, facility location, time hospitalized, stage and grade were also significantly associated with time to radiation therapy in univariable analyses In a multivariable model, adjusting for these factors as well as year of diagnosis, income, insurance status, and Charlson-Deyo score, surgical approach overall did not retain statistical significance (p=0.07); however, laparoscopic approach remained associated with quicker times to radiation (robotic HR 1.00 [95% CI 0.96, 1.04]; laparoscopic HR 1.06 [95% CI 1.01, 1.13]). Age, facility location, time hospitalized, stage, and grade remained statistically significant after adjustment (Table 3). After adjusting for all other covariates, being younger and having worse disease status as measured by stage and grade are associated with longer wait times to radiation.
Table 2:Time to radiation therapy percentiles for endometrial cancer patients by surgical approach.
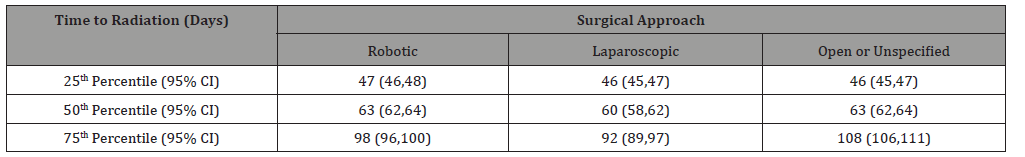
Table 3: Unadjusted and adjusted hazard ratio estimates.
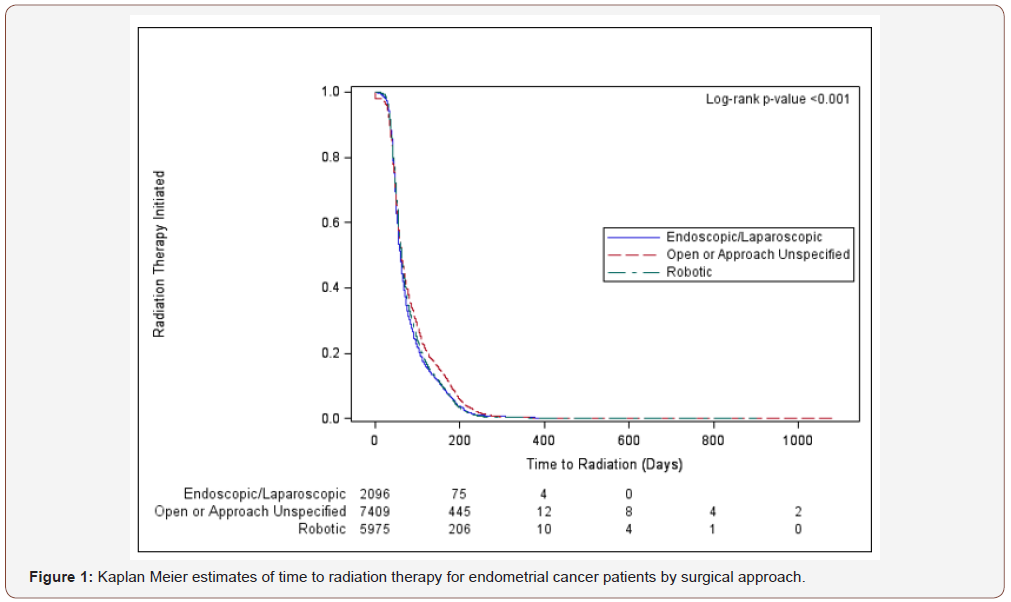
Discussion
To the best of our knowledge, this is the first report in a large database to have studied the impact of surgical technique on time to initiation of adjuvant RT in patients with endometrial cancer. The study’s main findings are that younger age, later stage at diagnosis, higher grade of tumor at diagnosis and laparotomy were all associated with longer surgery-to-radiation intervals. Laparotomy resulted in delays to initiation of RT when compared with roboticassisted laparoscopy and conventional laparoscopy.
The majority of the existing literature affirming the clinical significance of delays in adjuvant RT after surgery is in patients with locally advanced breast cancer. The largest and most recent metaanalysis evaluating the effect of waiting times for postoperative RT on breast cancer patient outcomes included a review of 34 publications encompassing a total of 79,616 patients [16]. This study reported that delays in post-lumpectomy adjuvant RT are significantly associated with risk of local recurrence in patients with locally advanced disease [16].
Within the gynecologic oncology literature, most of the studies evaluating the clinical impact of time to adjuvant therapy after surgery are in patients with cervical cancer. A retrospective study performed in Thailand identified 125 patients over 29 years who underwent radical hysterectomy for stage IA2 or IB1 squamous cervical cancer who received either adjuvant radiation therapy or adjuvant concurrent chemoradiation [17]. The investigators concluded that a time interval greater than 4 weeks between surgery and adjuvant therapy was associated with a worse recurrence-free survival [17]. Jhawar S, et al [18] performed a large-scale multi-institutional analysis of 3051 patients with early cervical cancer undergoing radical hysterectomy followed by adjuvant chemoradiation using the National Cancer Database [18]. The results of this study indicate that a delay of greater than 7 weeks from surgery to initiation of chemoradiation is associated with worse survival outcomes [18]. In a retrospective study of 226 patients with early stage cervical cancer, You KY, et al [19] similarly investigated the clinical significance of interval from surgery to adjuvant therapy by first dividing patients into two cohorts by FIGO staging – IA2-IB1 and IB2-IIA [10]. They concluded that for patients with stage IB2-IIA disease, overall survival and disease-free survival were higher for patients who had an interval to adjuvant treatment less than 5 weeks, but no similar effect was observed for patients with earlier stage disease [19].
Very few studies specifically evaluate differences in clinical outcome due to delay to adjuvant radiation therapy in endometrial cancer patients. In a retrospective single-institution study by Ahmad et al in 1995, investigators reviewed charts for 195 endometrial cancer patients collected over 22 years who all received adjuvant whole pelvic external beam radiation therapy following surgical staging [11]. Based on their findings, they concluded that a surgeryto- radiation delay of 6 weeks or greater was significantly associated with decreased disease specific survival [11]. This study, however, did not specifically evaluate surgical approach as a potential factor accounting for delays in treatment. A similar retrospective singleinstitution study by Cattaneo, et al in 2016 reviewed 308 patient charts over 22 year period which showed a surgery-to-radiation delay of 9 weeks or greater to be an independent predictor of recurrence as well as worse disease specific survival and overall survival in patients with endometrial cancer [13].
To our knowledge only one study specifically explores surgical approach as a possible factor responsible for delays to initiation of adjuvant RT in endometrial cancer patients. In a retrospective single-institution study, Stahl et al compared 74 patients who underwent robotic-assisted laparoscopic hysterectomy for staging for stage I-II endometrial carcinoma with 63 patients who underwent total abdominal hysterectomy for the same indication [20]. They concluded that while the average time interval from surgery to radiation oncology consultation was similar between the two groups (30 days), that patients who underwent roboticassisted laparoscopic hysterectomy had on average a one week delay in actual initiation of treatment due to longer vaginal cuff healing time [20].
In direct comparison, our study found that laparotomy leads to longer interval time to initiation of adjuvant therapy as compared to a minimally invasive approach. Possible reasons for delays to initiation of adjuvant RT in this cohort could include longer hospital stays, longer recovery time and increased risk of postoperative complications in patients undergoing laparotomy versus minimally invasive procedures. It is also possible that patients with worse pre-operative disease burden (higher grade and later stage disease) were more likely to be selected for laparotomy than minimally invasive approaches and therefore possibly also had worse pre-operative functional status which could contribute to longer recovery time and delay to initiation of adjuvant therapy.
The strengths of our study include utilization of a large-scale national database, which allowed for a much larger sample size. Additionally, because our cohort is multi-institutional our data is more generalizable when compared to single institution studies where there may be site-specific confounding variables with regard to surgical technique, clinical care, or follow-up that could affect results.
There are several limitations to our study that are inherent to the use of a national, hospital-based registry. Prior to 2010, information regarding mode of surgery was not recorded, so data for this study was limited to after 2010. Furthermore, the NCDB does not describe reasons why adjuvant RT may have been delayed nor does it provide specific information regarding adjuvant treatment regimens (i.e. whether adjuvant chemotherapy was needed, and adjuvant radiation was intentionally planned for after completion of chemotherapy). Those patients with advanced disease or earlystage high-risk histology that received adjuvant chemotherapy in addition to adjuvant RT might have in fact started their adjuvant treatment earlier than the results seen. Lastly, because we did not have information regarding initial treatment plans for these patients, we could not assess if there were patients in our cohort who were supposed to receive RT but were lost to follow-up, changed providers, or passed away before treatment had begun. As is the case with other published studies utilizing NCDB, we recognize that there are limitations with using this data and hence the conclusions drawn from this study warrant additional research and investigation.
Thus, younger age, higher stage or grade at diagnosis, and laparotomy all contribute to longer delays to initiation of adjuvant radiation therapy after surgery. While prospective studies are needed to better assess how delays in initiation of adjuvant RT affect clinical outcomes, these studies would be difficult to perform given our desire to have every patient treated as expeditiously as possible. As such, efforts should be made by individual institutions by means of quality improvement studies to identify potential sitespecific causes accounting for delays to initiation of adjuvant RT. Subsequently, attention should be given to addressing identified barriers to receiving care and ensuring streamlined and timely follow-up for all patients. Gynecologic oncologists and radiation oncologists are encouraged to remain cognizant of the timing between surgery and adjuvant RT for local control and initiate therapy at the soonest, safest time point.
Acknowledgement
None.
Conflict of Interest
Authors declare no conflict of interest
References
- Siegel RL, Miller KD, Jemal A (2017) Cancer statistics. CA Cancer J Clin 68(1):7-30.
- Aalders J, Abeler V, Kolstad P, Onsrud M (1980) Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: clinical and histopathologic study of 540 patients. Obstet Gynecol 56: 419-427.
- Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, et al. (2014) A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 92(3): 744-751.
- Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. (2006) Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol 24(1): 36-44.
- Janda M, Gebski V, Davies LC, Forder P, Brand A, et al. (2017) Effect of Total Laparoscopic Hysterectomy vs Total Abdominal Hysterectomy on Disease-Free Survival Among Women with Stage I Endometrial Cancer: A Randomized Clinical Trial. JAMA 317(12):1224-1233.
- Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, et al. (2009) Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol 27(32): 5331-5336.
- Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, et al. (2012) Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol 30(7): 695-700.
- Park DA, Lee DH, Kim SW, Lee SH (2016) Comparative safety and effectiveness of robot-assisted laparoscopic hysterectomy versus conventional laparoscopy and laparotomy for endometrial cancer: A systematic review and meta-analysis. Eur J Surg Oncol 42(9): 1303-1314.
- Park HK, Helenowski IB, Berry E, Lurain JR, Neubauer NL (2015) A comparison of survival and recurrence outcomes in patients with endometrial cancer undergoing robotic versus open surgery. J Minim Invasive Gynecol 22(6): 961-967.
- Mäenpää MM, Nieminen K, Tomás EI, Laurila M, Luukkaala TH, et al. (2016) Robotic-assisted vs traditional laparoscopic surgery for endometrial cancer: a randomized controlled trial. Am J Obstet Gynecol 215(5): 588.
- Ahmad NR, Lanciano RM, Corn BW, Schultheiss T (1995) Postoperative radiation therapy for surgically staged endometrial cancer: impact of time factors (overall treatment time and surgery-to-radiation interval) on outcome. Int J Radiat Oncol Biol Phys. 33(4): 837-842.
- Fabrini MG, Gadducci A, Perrone F, La Liscia C, Cosio S, et al. (2012) Relationship between interval from surgery to radiotherapy and local recurrence rate in patients with endometrioid-type endometrial cancer: a retrospective mono-institutional Italian study. Anticancer Res 32(1): 169-173.
- Cattaneo R, Hanna RK, Jacobsen G, Elshaikh MA (2014) Interval between hysterectomy and start of radiation treatment is predictive of recurrence in patients with endometrial carcinoma. Int J Radiat Oncol Biol Phys 88(4): 866-871.
- Raval MV, Bilimoria KY, Stewart AK, Bentrem DJ, Ko CY (2009) Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol 99(8): 488-490.
- Mohanty S, Bilimoria KY (2014) Comparing national cancer registries: The National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. J Surg Oncol 109(7): 629-630.
- Gupta S, King WD, Korzeniowski M, Wallace DL, Mackillop WJ (2016) The effect of waiting times for postoperative radiotherapy on outcomes for women receiving partial mastectomy for breast cancer: a systematic review and meta-analysis. Clin Oncol (R Coll Radiol). 28(12): 739-749.
- Hanprasertpong J, Jiamset I, Geater A, Leetanaporn K, Peerawong T (2017) Impact of time interval between radical hysterectomy with pelvic node dissection and initial adjuvant therapy on oncological outcomes of early stage cervical cancer. J Gynecol Oncol 28(4): e42.
- Jhawar S, Hathout L, Elshaikh MA, Beriwal S, Small W Jr, et al. (2017) Adjuvant chemoradiation therapy for cervical cancer and effect of timing and duration on treatment outcome. Int J Radiat Oncol Biol Phys 98(5): 1132-1141.
- You KY, Zhou XH, Jiang YH, Bi ZF, Liu YM, et al. (2018) The selection of time interval between surgery and adjuvant therapy in early stage cervical cancer. Int J Gynecol Cancer 28(7): 1325-1332.
- Stahl JM, Park HS, Silasi DA, Azodi M, Damast S (2016) Influence of robotic-assisted laparoscopic hysterectomy on vaginal cuff healing and brachytherapy initiation in endometrial carcinoma patients. Pract Radiat Oncol 6(4): 226-232.
-
Shannon Tomita, Melissa Schwartz, Valentin Kolev, Samantha Raymond, Jessica Overbey, et al. The Effect of Primary Surgical Technique for Treatment of Endometrial Cancer and Timing of Adjuvant Radiation Therapy. W J Gynecol Women’s Health. 3(1): 2019. WJGWH. MS.ID.000553.
Surgical technique, Endometrial cancer, Radiation therapy, Gynecologic malignancy, Radiotherapy, Chemotherapy, Laparotomy, Laparoscopy, Morbidity, Surgery
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






