 Research Article
Research Article
Importance of Sonographic Endometrial Morphology in Detecting Hyperplasia and Carcinoma
M Terrani1, BM Petrikovsky2*, K Zakashansky3, M Culotta1 and A Dillon1
1Garden Obstetrics and Gynecology, Long Island, USA
2Chairman of Obstetrics and Gynecology, Nassau University Medical Center, USA
2Mount Sinai Medical Center, Icahn School of Medicine, USA
BM Petrikovsky, Professor and former Chairman of Obstetrics and Gynecology, Nassau University Medical Center, USA.
Received Date: May 29, 2019; Published Date: June 03, 2019
Introduction
In 2008, the American College of Obstetricians and Gynecologists (ACOG) put together a special committee to produce recommendations on the role of transvaginal sonography to evaluate endometrium in postmenopausal women [1]. Transvaginal ultrasonography usually is sufficient for an initial evaluation of postmenopausal bleeding if the ultrasound images reveal a thin endometrial echo (less than or equal to 4 mm), given that an endometrial thickness of 4mm or less has a greater than 99% negative predictive value for endometrial value for endometrial cancer [1].
However, certain types of endometrial carcinoma e.g., type II can present with endometrial thickness of less than 4 mm [1]. The International Endometrial Tumor Analysis (IETA) group was formed in Chicago at the World Congress of Ultrasound in Obstetrics and Gynecology in 2008 with the aim of agreeing on terms and definitions to describe ultrasound findings in the uterine cavity.
We present retrospective analysis of 1,842 patients in whom we compare endometrial thickness and appearance (morphology) with the results of clinically indicated endometrial biopsy results.
Material and Methods
This is a retrospective cohort study of 1,842 patients aged 45 – 76 years old who underwent transvaginal sonography between June 2008 and August 2018. Seven hundred and twelve patients were premenopausal, 1,130 patients – postmenopausal. Endometrial sampling was performed in 612 cases based on suspicious sonographic or clinical symptoms. Sonographic features of the endometrium and intrauterine lesions were described using IETA criteria [2].
In premenopausal patients, a sonographic examination was performed in the early proliferative phase and in postmenopausal women on cyclic hormonal replacement therapy 5 – 10 days after the last progestin tablet. The uterus was scanned in the sagittal plane from cornu to cornu and in the (oblique) transverse plane from the cervix to the fundus.
For endometrial thickness assessment the calipers were placed at the level of the two-opposite endometrial-myometrial interfaces and the endometrium was measured where it appears to be at its thickest perpendicular to the midline. The measurement of the total double-layer thickness was reported in millimeters, rounded up to one decimal point. When intracavitary fluid is present, the thickness of both single layers was measured, and the sum is recorded. If the endometrium is thickened asymmetrically the largest anterior and posterior endometrial thickness was reported separately
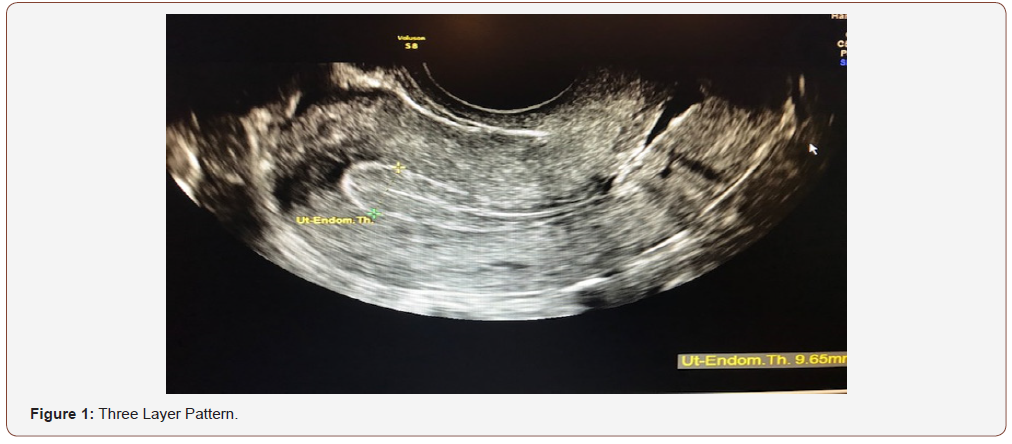
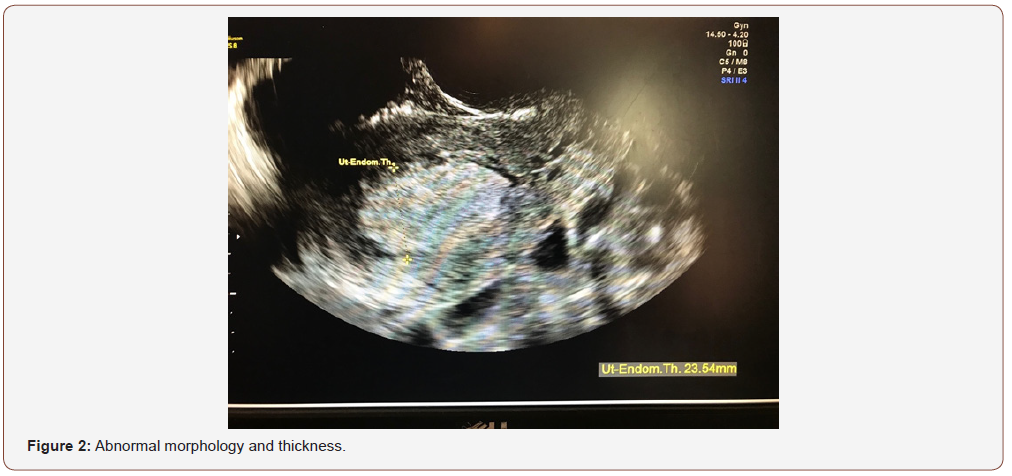
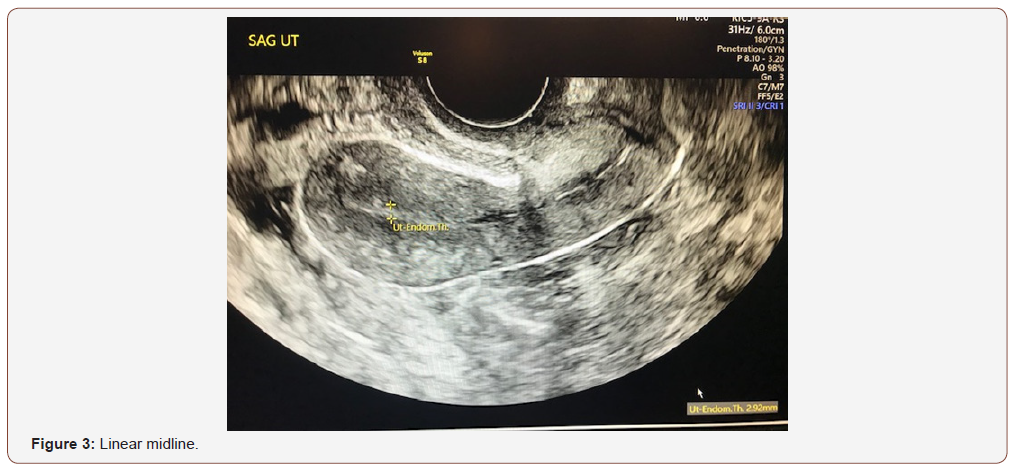
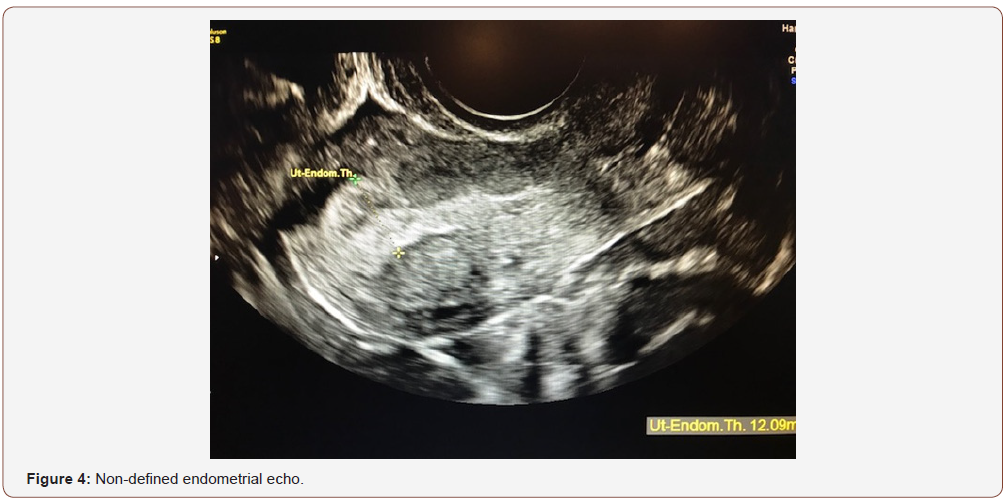
An evaluation of endometrial morphology included an assessment of endometrial echogenicity, the endometrial midline and the endometrial-myometrial junction. A ‘uniform’ endometrium included the three-layer pattern (Figure 1), as well as a homogenous hyperechogenic, hypoechogenic and is echogenic endometrium. The echogenicity is defined as ‘non-uniform’ if the endometrium appears heterogenous, asymmetrical or cystic (Figure 2). The endometrial midline was defined as ‘linear’ (Figure 3), if a straight hyperechogenic interface within the endometrium is visualized, as ‘non-linear’ if a waved hyperechogenic interface is seen, and as ‘irregular’ or as ‘not defined’ in the absence of a distinct interface (Figure 4). All studies were performed at the AIUM certified sonographic unit.
Statistical Analysis
Data analysis was performed with SPSS 21.0. Normality was evaluated using Shapiro-Wilk test. Standard X2 test and analysis of variance test were used to compare categorical and continuous variables. Variables that were found to be different between groups (P<.05) in the bivariate analysis were entered to the multivariable logistic regression model. The model was tested for goodness of fit. Differences were considered significant when the P value was <.05.
Results
Endometrial pathology was detected in 69 out of 612 patients who underwent biopsy for accepted clinical indications, polyps in 36, simple hyperplasia in 25, complex hyperplasia in 13, and endometrial cancer in [5].
Comparison of endometrial thickness and morphology with biopsy results revealed the following: Simple hyperplasia has increased thickness in 9 out of 15 cases and abnormal appearance in 6 (non-uniform echogenicity, lack of 3-layer pattern); in cases of complex hyperplasia and cancer, endometrial thickness was abnormal in 15 out of 18 cases (83%) and morphology in all cases (heterogeneous, asymmetrical or cystic).
Discussion
Cancer of the endometrium is the most common type of gynecologic cancer in the United States. In 2007, an estimated 61,380 new cases of uterine cancer were diagnosed, and an estimated 10,920 deaths occurred [3]. The earliest reports comparing transvaginal ultrasonography with endometrial sampling consistently found that an endometrial thickness of 4-5 mm or less reliably excluded endometrial cancer [4,5]. However, type II endometrial carcinomas and endometrial cancer precursors had been detected in women with endometrial thickness of less than 3 mm [1].
Using a 4mm endometrial echo as a cut-off value, transvaginal ultrasonography has high negative predictive value. However, a thickened endometrial echo is not diagnostic of any particular pathology [1]. Furthermore, a thin endometrial echo does not reliably exclude type II endometrial cancer (uterine papillary serous, mucinous, or clear cell carcinomas) [6].
The presence of endometrial cancer in polyps, although rare, have been reported as well.7 In view of the above, we conducted a retrospective study comparing the value of endometrial thickness versus morphology in recognizing endometrial pathology.
We conclude that abnormal morphology matters more than abnormal thickness in detection of precancerous and cancerous lesions of the endometrium. The loss of trilinear appearance in premenopausal women and monolayer in postmenopausal is most indicative of endometrial pathology. This conclusion doesn’t neglect the significance of endometrial thickness in screening for endometrial pathology but rather add another important parameter – endometrial morphology.
The weaknesses of the study are its retrospective nature and the fact that endometrial biopsy had been performed in a selected number of indicated cases. This allows the possibility of some cases of endometrial pathology to remain undetected. The strength of the study is a large number of participants and the fact that all sonographic examinations were performed by AIUM certified technicians using uniform IETA protocol. Another strong point of the study is that it represents one of the few papers comparing endometrium thickness and appearance (morphology) in predicting endometrial pathology.
Our results confirm IETA statement that, “Endometrial thickness is certainly important as a predictor of pathology, but endometrial morphology may prove just as useful. In particular, it might be useful in premenopausal patients, in whom endometrial thickness has been shown to be of limited value as a predictor of abnormal findings [2,8].
Attention to sonographic endometrial morphology may be especially important to premenopausal patients with risk factors for developing malignancy, unopposed estrogen, diabetes, nulliparity, and obesity, characterized by a BMI > 25 kg/m2, among others. Transvaginal assessment of endometrial thickness can be used with the same cutoff in postmenopausal women estrogen replacement, but assessment of endometrial thickness is less useful in the premenopausal population [9].
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- (2018) The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. ACOG Committee Opinion. Number 734.
- Leone FP, Timmerman D, Bourne T, Valentin L, Epstein E, et al. (2010) Terms, definitions and measurements to describe the sonographic features of the endometrium and intrauterine lesions: a consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Ultrasound Obstet Gynecol 35(1): 103-112.
- (2017) American Cancer Society. Cancer facts and figures. Atlanta, USA.
- Varner RE, Sparks JM, Cameron CD, Roberts LL, Soong SJ (1991) Transvaginal sonography of the endometrium in postmenopausal women. Obstet Gynecol 78(2): 195-199.
- Granberg S, Wikland M, Karlsson B, Norström A, Friberg LG (1991) Endometrial thickness as measured by endovaginal ultrasonography for identifying endometrial abnormality. Am J Obstet Gynecol 164 (1 Pt 1): 47-52.
- Wang J, Wieslander C, Hansen G, Cass I, Vasilev S, et al. (2006) Thin endometrial echo complex on ultrasound does not reliably exclude type 2 endometrial cancers. Gynecol Oncol 101(1): 120-125.
- Ferrazzi E, Zupi E, Leone FP, Savelli L, Omodei U, et al. (2009) How often are endometrial polyps malignant in asymptomatic postmenopausal women? A multicenter study. Am J Obstet Gynecol 200(3): 235. e1-e6.
- Dijkhuizen FP, Brölmann HA, Potters AE, Bongers MY, Heinz AP (1996) The accuracy of transvaginal ultrasonography in the diagnosis of endometrial abnormalities. Obstet Gynecol 87(3): 345-349.
- Trimble CL, Method M, Leitao M, Lu K, Ioffe O, et al. (2012) Management of endometrial precancers. Obstet Gynecol 120(5): 1160-1175
-
M Terrani, BM Petrikovsky, K Zakashansky, M Culotta, A Dillon. Importance of Sonographic Endometrial Morphology in Detecting Hyperplasia and Carcinoma. W J Gynecol Women’s Health. 2(3): 2019 WJGWH.MS.ID.000540.
Sonographic, Endometrial, Morphology, Hyperplasia, Carcinoma, Obstetricians, Transvaginal, Endometrium, Postmenopausal
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






