 Research Article
Research Article
Utilization of Raw, Dehullled, Autoclaved and Soaked Pea Pisum Sativum Seed Meals as Replacement for Fishmeal in Practical Diet Formulation for Juvenile Sea Bass in a Recirculating System
Erlinda S Ganzon Naret, University of the Philippines Visayas, College of Fisheries & Ocean Sciences, Institute of Aquaculture, Iloilo, Philippines.
Received Date: February 15, 2019; Published Date: March 18, 2019
Abstract
A 60-day feeding experiment on sea bass, Lates calcariform was conducted at the UPV Multi- Species Hatchery from September 23 - November 21, 2017. Healthy juveniles with an average initial weight of 0.09g were randomly stocked at twenty-five fish per tank at three replicates per treatment in fifteen 100-l conical tanks connected to a recirculating system under a photoperiod of 12h light and 12h dark. Five experimental diets were formulated to be isocaloric (40%) and isolipidic (10%) consisting of a Control Diet (without green pea); raw green pea, RGP (Diet 1); dehulled green pea, DGP (Diet 2); autoclaved green pea, AGP (Diet 3 ) and soaked green peas, SGP (Diet 4) to replace about 10-11% of the total protein in the diets. The percent weight gain (% WG) of sea bass fed control diet (1544±2.60%) was significantly (P<0.05) higher than diets with RGP, SGP and DGP, however fish fed AGP diets was not significantly different from those of the control group (P > 0.05). No significant difference (P>0.05) was found in the specific growth rate (%SGR) among the groups of fish fed control, AGP and DGP diets. Similarly, the SGR were not also markedly different for fish fed DGP and SGP diets, nevertheless the values were the lowest for the RGP diets. The best feed conversion ratio (FCR) and protein efficiency ratio (PER) were observed in sea bass fed control diet at 1.74 and 1.88 respectively. The highest survival rate at the end of the 60-day feeding period was observed for DGP diet (85.33%) which was comparable to those obtained with the other diets (AGP, SGP and Control), however the value was lowest for RGP diet (73.33%). Based on these results FM based diets with AGP and DGP have similar effects on the growth performance and survival of juvenile sea bass, L calcariform. It is quite clear that autoclaving and dehulling should be effectively used, not only for improving the nutritional quality of P. sativum, but also for reducing its antinutritional components as dietary protein source for sea bass.
Keywords: Green peas; Sea bass; Recirculating system; Dehulled peas; Growth
Introduction
Fish meal (FM) is the major component in fish diets due to its good amino acid profile, high digestibility and palatability. However due to its high demand and limited supply, FM resulted in a massive increase in prices, thus several investigators had to look for alternative plant protein sources for use by the fish growers in aquaculture industry [1-3]. Previous studies in fish had been carried out using plant protein components to partially or completely replace FM in for different fish species owing to its nutritional quality, availability and low cost [4-9].
Numerous studies have described peas as potential dietary protein ingredient in marine fishes and crustaceans such as European sea bass, Dicentrarchus labrax [10]; blue shrimp, Litopenaeus stylirostris [11]; juvenile pacific white shrimp, Litopenaeus vannamei [12]; milkfish, Chanos-chanos Forsskal [13]; juvenile tiger shrimp, Penaeus monodon [14] and in Atlantic salmon, Salmo salar [15]. Francis et al. [18] and Sharma et al. [17] reported the use of grain legumes as feed ingredients is limited due to the presence of trypsin inhibitors (TIA), phytic acid, tannins and saponins which decrease the nutritive value of the legumes, thus reducing food intake and nutrient utilization in animals. In fact, several researchers had advocated the various processing methods such as heat treatment and other physical methods to eliminate the anti-nutritional components in legumes [18,19].
The objectives of this study were to process P Sativum using the different physical and heat treatment methods in order to assess the same the use of these raw and processed green pea seed meals to partially replace fish meal as protein sources in formulated diets based on the growth performance and survival of sea bass in the recirculating system.
Materials and Methods
Preparation and processing of green pea seeds
Dry and mature seeds of green peas were obtained from the local market in Iloilo City, Philippines. The seeds were placed in trays made of bamboo splints and willowed using electric fan to remove the dusts and dirt’s. They were kept in sealed plastic bags and stored in the refrigerator to prevent from molds and insect infestation. Three processing methods were employed for P. sativum seeds which include soaking, dehulling and autoclaving and was carried out at the Wet Laboratory of the Institute of Aquaculture, College of Fisheries and Ocean Sciences, UP Visayas, Miag-ao, Iloilo.
About 5kg of RGP seeds were soaked in distilled water at a ratio of 1:10 for 12 h at room temperature, dried and milled to pass through the 60-mesh sieve. Another portion of the seeds were dehulled using mortar and pestle then dried before milling. The third batch of seeds were spread in the aluminum trays at a depth of 1-2cm and heat treated in the autoclave at 15psi for 60 min. at 121°C. The samples were dried, cooled and milled to pass through a 60 mm sieve while the remaining portion of the whole unprocessed RGP seeds, about 5kg were milled into powder form.
Samples of raw and processed green pea meals were analyzed for proximate composition following the procedure as described by the Association of Official Analytical Chemists (AOAC 2000) as shown in Table 1. In the present study, the amino acid composition of the test ingredients (Table 2) was determined by hydrolyzing the 5.0 mg of protein sample using nor leucine as an internal standard added to the HCl and was carried out using the high-performance liquid chromatography (HPLC) and analyzed by the Feeds & Foods Nutrition Research Center at the Pukyong National University in Busan, South Korea.
Table 1: Proximate composition of raw and processed green pea seeds (g/100g DM)*.
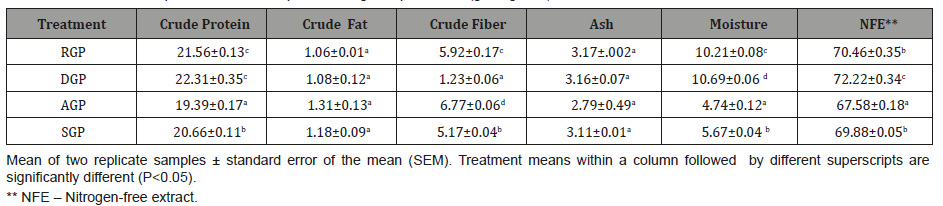
Table 2: Amino acid content (g/100 g dry weight) of raw and processed green pea meals as dietary protein sources.
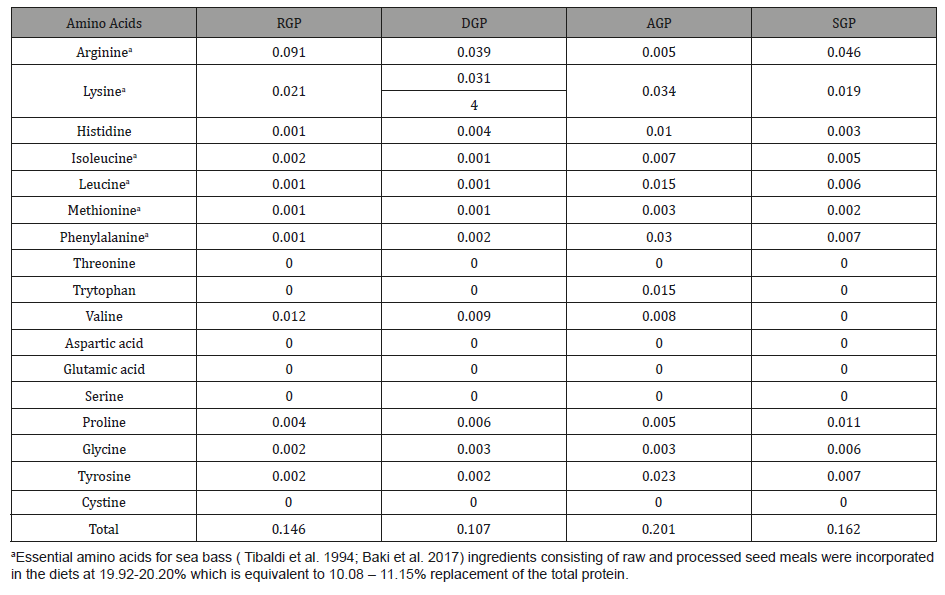
Table 3: Composition (g/100g diet) and proximate analyses (%) of experimental diets.
Five experimental diets (Table 3) were formulated to be isonitrogenous and isolipidic (40% protein and 10% fat). The basal diet contained fish meal with equal amounts of shrimp meal, squid meal, soybean meal and Gracilaria sp. added into each diet. The test ingredients consisting of raw and processed seed meals were incorporated in the diets at 19.92-20.20% which is equivalent to 10.08 - 11.15% replacement of the total protein. Bread flour was used as a binder and the amount was adjusted when RGP, DGP, AGP and SGP were added to maintain similar dietary protein contents in the diets. The dry ingredients were mixed carefully prior to the addition of vitamin, mineral and BHT (Butyl hydroxytoluene). Corn oil and cod liver oil (1:1) were added to the mixture. Diets were prepared following the FDS Manual (1994) and the dry extruded pellets were placed in plastic containers and stored at 40C before feeding. Proximate analyses of experimental diets were determined by the standard methods as described by AOAC (2000).
Fish and experimental condition
The experiment was conducted in a recirculating system at the University of the Philippines Visayas Multi-Species Hatchery, Miagao, Iloilo. Juvenile sea bass were obtained from SEAFDEC, Tigbauan, Iloilo. Fish were acclimated to the recirculation system at least 2 weeks before the trial. Healthy juveniles (Table 4) of similar sizes (initial body weight, IBW = 0.09±0.01 g) were randomly distributed at a stocking rate of 25 fish per tank into 15 -100L conical tanks. Aeration was supplied to each tank 24h daily.
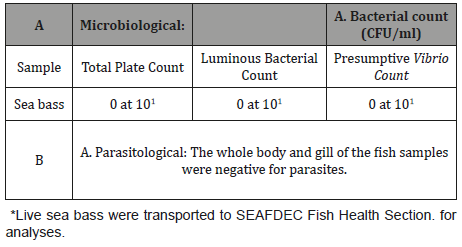
Table 4: Microbiological and parasitological results of juvenile sea bass* used for the experiment.
Three tanks were randomly assigned to each diet group for a 60-day feeding experiment. Feed was given thrice daily (09.00; 13:00 and 16:00h) at a feeding rate of 25% and was adjusted every 15days. Seawater was monitored for its temperature of 25-280C using a thermometer; dissolved oxygen was measured by YSI DO meter and it ranged from 6.5 to 7.4 ppm; pH of 7.3-7.7 and salinity which range from 28-32ppt were determined using the pH meter and refractometer (Atago) respectively. The values for ammonia – nitrogen (NH3-N) and nitrite-nitrogen (NO2-N) were measured from the water samples taken inside the tanks every 15 days and the values were within the ranges appropriate for the growth of sea bass. Uneaten feeds and feces were siphoned, and mortality was recorded daily. All the surviving fish in each tank were counted and group weighed using a precision balance to the nearest 0.001 g (Shimadzu, TXB 522L) every 15 days to adjust the feeding ration.
Calculation and Statistical analysis
Five experimental diets were used in the feeding experiment. The diet was tested in each tank at three replicates. All the data were analyzed by a one-way ANOVA for a completely randomized design (CRD) using a Statistical Analysis Software Program of SPSS Version 18. The Duncan’s Multiple Range Test (DMRT) was used to determine the differences between the treatment means. Survival was computed using the arcs in square root. And results were considered significant at a level of P<0.05. Growth and feed utilization parameters were calculated as follows:
SGR (%day-1) = (In Wf – In Wi) x 100/T and
WG (%) = (Wf – Wi)/Wi x 100
where Wf is the final weight (g), Wi is the initial weight and T is the culture period (days).
FCR = dry feed intake/wet weight gain
PER = weight gain/protein intake.
Survival (%) = Final number of fish/Initial number of fish x 100.
Results
The proximate composition of raw and processed green pea seeds are presented in Table 1. The moisture contents between the raw and processed peas were significantly different (P<0.05) among treatments. Autoclaving and soaking significantly (P<0.05) decreased the moisture content at 4.74 and 5.67% respectively. It was noted that the ash content of AGP and SGP were considerably low which ranged from 2.79 -3.11% as compared with DGP and RGP which ranged from 3.16 – 3.17%, however no significant (P>0.05) differences were observed among the treatments. The crude protein level for DGP was significantly higher (P<0.05) as compared with two other treatments at 19.39% and 20.66 % for AGP and SGP respectively and was not significantly (P>0.05) different from the value obtained for RGP (21.56%). The highest crude fat (1.31%) was obtained in AGP and was not significantly (P>0.05) different among the RGP, DGP and SGP values. Based on the crude fiber content of raw and processed pea samples, DGP (1.23) showed the lowest significant (P<0.05) value followed by RGP and SGP with the highest value obtained for AGP (6.77). Among the processing methods such as autoclaving and soaking, dehulling significantly (P<0.05) improved the NFE of GPs in the present study.
Table 2 shows the amino acid composition of raw and processed GPs. The total free amino acids of the given four GPs samples ranged from 0.107 – 0.201 g/100 g dry weight. Also, the essential amino acid content of all test GP samples was generally low, except for the arginine content in RGP which was 0.091 mg/100g, however the value was not significantly (P>0.05) different from DGP, AGP and SGP at 0.039, 0.005 and 0.046 mg/100g respectively. The proximate analyses of control and experimental diets (Diets 1-4) is shown in Table 3. All diets were formulated to be isonitrogenous and isolipidic (40% protein and 10% fat). Results showed that crude protein ranged from 39.88 to 41.12% while the values for crude fat were 10.20 to 10.83%.
At the start of the feeding trial juvenile sea bass were analyzed for microbiological and parasitological examination at the Fish Health Section, SEAFDEC, AQD, Tigbauan and the samples were found to be free from bacterial diseases and parasites (Table 4). Table 5 shows the growth response and survival of sea bass. after 60 days of culture period.
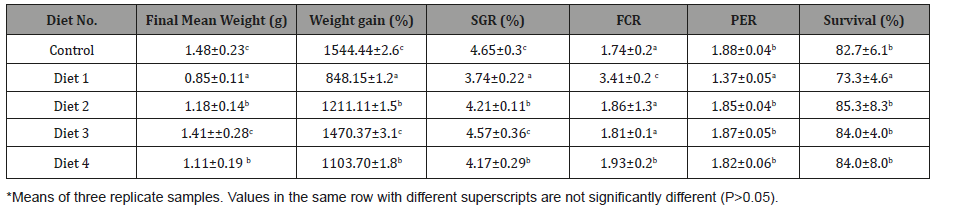
Table 5: Growth and survival of juvenile L. calcarifer fed diets containing protein from raw and processed GPs for 60days in a recirculating system (initial weight of 0.09±0.01g)*.
Discussion
The highest moisture content was observed for the DGP (10.69%), which was consistent with the findings obtained for the dehulled raw mungbean flour at 10.14% [20]. It was also apparent that the moisture content in the AGP was significantly decreased as a result of processing the seeds at high temperature of 210oC for 60 min. Thermal processing may improve the nutritional value and reduce the level of anti-nutritional components. in GP meals.
Results of the proximate analyses showed that protein content of DGP was significantly higher from those values obtained in SGP and AGP. These results are comparable with the findings of [21,22] in horsegram seeds [23] found that dehullling significantly improved the protein digestibility of green gram, cowpea, lentil and chickpeas at 2.2, 5.1, 13.2 and 16.7% respectively, which could be explained due to the removal of hull portion and concentration of endosperm after dehulling. It was noted that the NFE content in the dehulled sample was significantly higher among the various samples, this can be attributed possibly due to the removal of the hulls.
The result indicates that the different processing methods did not affect the fat content of green peas which is due to the insoluble nature of fat. These observations are in agreement with those reported by [24] in lentils (Lens culinaris) and [25] for lima beans (Phaseolus lunatus).
Data presented in Table 2 shows the amino acid composition of raw and processed green peas. Green pea’s protein contains relatively low concentration of essential and non- essential amino acids as compared to the different varieties of P. sativum tested by [27] in Asia and with the FAO/WHO Reference Pattern [26]. There were no significant changes in the free amino acids among all the GP samples. Like other legumes, P. sativum seeds are deficient in sulphur containing amino acids (methionine and cysteine) and to a lesser extent tryptophan and threonine which was similar to the results obtained by [28 in Phaseolus seeds. Dehulling and autoclaving caused a slight increase in lysine, whereas there was no effect (P>0.05) on the availability of arginine.
In the present study, the growth performance in terms of final weight, weigh gain (%) and SGR of fish fed Diet 3 was comparable to those fish fed the Control Diet without green peas. This could be the result that green peas which were incorporated at 20.80% as shown in Table 3 were heat-treated in an autoclave at 121oC for 15 psi for 60 min which eliminated the condensed tannin content prior to its addition in sea bass diets. These results conform with the reports of [29] on the mirror carp fed diets containing 10-30% heated Vicia peregrina seeds. The presence of tannin lowers the nutritive value and reduces the biological availability of proteins, carbohydrates, amino acids, vitamins and minerals in legumes [30,31]. The growth of seabass offered the raw green pea-based diet (Diet 1) had significantly lower growth performance and this can be attributed possibly to the presence of growth inhibiting substances present in raw peas. This observation is also consistent with the findings of [13] for milkfish. In this study, processing such as dehulling and soaking had no significant effect on the growth and survival of L. calcariform, this may be due on the variety of peas used, although the nutritional value of green peas was slightly improved. Diet 1 containing raw green peas resulted in significantly poorer feed utilization for fish as evidenced with the values obtained for the FCR and PER as compared with the other treatments, this may be attributed to the presence of anti-nutrients in the unprocessed meal sample added to the diet. Akanje et al [32] found that the FCE and PER were markedly reduced in broiler chickens fed with raw cowpea. Although green peas are considered to contain anti-nutritive factors, the survival rate is high using the different processed peas (Diets 2-4) in the formulation for sea bass diets [33-36]. Fish accepted the diets readily without adverse effects on the growth performance.
This study demonstrated that processed GP meals can partially replace fish meal as a protein source up to 20% in diets with no adverse effects on the growth performance and survival of L. calcariform. It is also recommended that P. sativum should always be heat-treated (autoclave) to retain its nutritive value and enhance the feed utilization in fish. The anti-nutritional factors present in the raw green peas were responsible for the poorest growth and feed intake during the feeding experiment. Considering that legumes contain relatively low amino acids, therefore raw and processed green pea meals incorporated in sea bass diets should be supplemented with essential amino acids if the total replacement of protein is more than 10%.
Acknowledgement
The author would like to thank the University of the Philippines Visayas, Miag-ao, Iloilo for the Research Grant; J.A. Octaviano and R. Escorpion for their technical assistance and, Pukyong National University, Busan, South Korea for the amino acid analysis.
Conflict of Interest
No conflict of interest.
References
- Tacon AGJ, Metian M (2009) Fishing for Feed or Fishing for Food: Increasing Global Competition for Small Pelagic Forage Fish. Ambio 38(6): 294-302.
- Figueiredo Silva C, Lemme A (2014) Current Challenges and Opportunities in Amino Acid Nutrition of Salmonids. International Aquafeed 17: 16-19.
- Radhakrishnan S, Bhavan PS, Seenivasan C, Muralissankar T (2016) Impact of Fishmeal Replacement with Arthrospira platensis on Growth Performance, Body Composition and Digestive Enzyme Activities of the Freshwater Prawn, Macrobrachium rosenbergii. Aquaculture Research 3: 35-44.
- Belal IEH (1999) Replacing dietary corn with barley seeds in Nile tilapia Oreochromis niloticus (L) feed. Aquaculture Research 30(4): 265-269.
- Davis DA, Arnold CR (2000) Replacement of Fishmeal in Practical Diets for the Pacific White Shrimp, Litopenaeus vannamei. Aquaculture 185(3-4): 291-298.
- El Sayed AM (1994) Evaluation of Soybean Meal, Spirulina Meal and Chicken Offal Meal as Protein Sources for Silver Seabream (Rhabdosargus sarba) Fingerlings. Aquaculture 127(2-3): 169-17.
- Gallagher ML (1994) The Use of Soybean Meal as a Replacement for Fish meal in Diets for Hybrid Striped bass (Morone saxatilis x Morone chrysops). Aquaculture 126(1-2): 119-127.
- Khan MSK, Siddique MAM, Zamal H (2013) Replacement of Fish meal by Plant Protein Sources in Nile tilapia (Oreochromis niloticus) Diet: Gowth Performance and Utilization. Iranian Journal of Fisheries Sciences 12(4): 864-872.
- Ng WK, Wee KL (1989) The Nutritive Value of Cassava Leaf Meal in Pelleted Feed for Nile tilapia. Aquaculture 83(1-2): 45-58.
- Gouveia A, Davies SJ (1998) The Nutritional Evaluation of Pea Seed Meals in Diets for Juvenile European Sea bass (Dicentrarchus labrax). Aquaculture 182: 183-193.
- Cruz Suarez, LE, Ricque Marie D, Tapia Salazar M, Mcallum IM, et al. (2001) Assessment of Differently Processed Feed Pea (Pisum sativum) Meals and Canola Meal (Brassica sp.) in Diets for the Blue Shrimp (Litopenaeus styliirostris). Aquaculture 196: 87-104.
- Davis DA, Arnold CR, Mc Calllum IM (2002) Nutritional Value of Feed Peas (Pisum sativum) in Practical Diet Formulation for Litopenaeus vannamei Aquac Nutr 8(2): 87-94.
- Borlongan IG, Eusebio PS, Welsh T (2003) Potential of feed pea (Pisum sativum) meal as a protein source in practical diets for milkfish (Chanos chanos Forsskal). Aquaculture 225(1-4): 89-98.
- Bautista Teruel MN, Eusebio PS, Welsh TP (2003) Utilization of feed peas Pisum sativum meal as a protein source in practical diets for juvenile tiger shrimp Penaeus monodon. Aquaculture 225(1-4): 121-131.
- Overland M, Sorensen M, Stobakken T, Penn MH, Krogdahl A (2009) Pea Protein Concentrate Substituting Fish meal or Soybean meal in Diets for Atlantic salmon (Salmo salar)- Effect on Growth Performance, Nutrient Digestibility, Carcass Composition, Gut Health and Physical Feed Quality. Aquaculture 288(3-4): 305-311.
- Francis G, Makkar HPS, Becker K (2001) Anti-nutritional Factors Present in Plant Derived Alternate Fish Feed Ingredient and their Effects in Fish. Aquaculture 199(3-4): 197- 227.
- Sharma S, Kaur M, Goyal R, Gill BS (2011) Physical Characteristics and Nutritional Composition of Some New Soybean (Glycine max (L) Merrill) Genotypes. J Food Sci Technol 51(3): 551-7.
- Viola S, Mokady S, Arieli Y (1983) Effects of Soybean Processing Methods on the Growth of Carp (Cyprinus carpio). Aquaculture 32(1-3): 27-38.
- Eusebio PS (1991) Effect of Dehulling on the Nutritive Value of Some Leguminous Seeds as Protein Sources for Tiger Prawn, Penaeus monodon juveniles. Aquaculture 99(3-4): 297-308.
- Offia Olua BI, Madubuike UB (2015) The Dehulling Efficiency and Physico-chemical Properties of Pre-conditioned Mungbean (Vigna radiate (1) wilczek) Seeds and Flour. International Research Journal 6(1): 1-11.
- Pal RS, Bhartiya A, Arunkumar R, Kant L, Aditya JP, et al. (2016) Impact of Dehulling and Germination on Nutrient, Anti-nutrients and anti-oxidant properties in Horsegram. J Food Sci Technol 53(1): 337 -347.
- Sudha N, Mushtari Begum J, Shambulingappa KG, Babu CK (1995) Nutrients and some anti-nutrients in horsegram (Macrotyloma uniflorum (Lam.) Verdc.). Food Nutr Bull 16(1): 81-83.
- Ghavidel R, Prakash J (2007) The Impact of Germination and Dehulling on Nutrients, Anti-nutrients, in Vitro Iron and Calcium Bioavailability and in Vitro Starch and Protein Digestibility of Some Legume Seeds. LWT-Food Science and Technology 40(7): 1292-1299.
- Hefnawy TH (2011) Effect of processing methods on nutritional composition and anti-nutritional factors in lentils (Lens culinaris). Annals of Agricultural Science 56(2): 57-61.
- Oke MO, Sobowale SS, Ogunlakin GO (2013) Evaluation of the effect ofprocessing methods on the nutritional and anti-nutritional composition of two under-utilized Nigerian grain legumes. Pakistan Journal of Biological Sciences 16(24): 2015-20120.
- Iqbal A, Khalil AI, Ateeq N, Khan SM (2006) Nutritional Quality of Important Food Legumes. Food Chemistry 97(2): 331-335.
- FAO/WHO (1991) Food and Nutrition. In Protein Quality Evaluation Report of a Joint FAO/WHO Expert Consultation Food and Agriculture Organization of the United Nations, FAO, Rome 51: 1-66.
- Baudoin JP, Maquet A (1999) Improvement of protein and amino acid contents in seeds of food legumes. A case in Phaseolus. Biotechnol. Agron. Soc Environ 3(4): 220-224.
- Buyukcapar HK, Kamalak A (2010) Nutritive value of wild pea (Pisum elatius) seed as a dietary protein source for fingerlings of Mirror Carp (Cyprinus carpio). The Israeli Journal of Aquaculture- Bamidgeh 62(4): 272-280.
- Desphande SS, Cheryan M (1985) Evaluation of vanillin assay for tannin analysis of dry beans. J Food Sci 50(4): 905-908.
- Makkar HPS, Singh B, KK, Dawra KK (1987) Tannin-nutrient Interaction-a Review. Int J Anim Sci 2: 127-139.
- Akanji Am, Fasina OA, Ogungbesan A (2016) Effect of raw and processed cowpea on growth and haematological profile of broiler chickens. Bang J Anim Sci 45(1): 31-37.
- AOAC (2000) Official methods of analyses of AOAC International, 17th edn. Association of Official Analytical Chemists, Washington, DC.
- Baki B, Ozturk DK, Kerim M (2017) Determination of essential amino acid changes related to the growth of sea bass (Dicentrarchus labrax). Turkish Journal of Fisheries and Aquatic Sciences 17: 1425-1429.
- Feed Development Section (FDS) (1994) Feeds and Feeding of Milkfish, Nile tilapia, Asian Sea bass and Tiger Shrimp. SEAFDEC Aquaculture Extension Manual No. 21. SEAFDEC Aquaculture Department, Tigbauan, Iloilo, Philippines. Pp. 97.
- Tibaldi E, Tulli F, Lanari D (1994) Arginine Requirement and Effect of Different Dietary Arginine and Lysine Levels for Fingerlings Sea bass (Dicentrarchus labrax). Aquaculture 127(2-3): 207-218.
-
George E Rayment, David J Lyons. Trends in Soil Measurement Performance of Australasian Laboratories by Methods and Times. World J Agri & Soil Sci. 1(4): 2019. WJASS.MS.ID.000520.
-
Green peas, Sea bass, Recirculating system, Dehulled peas, Growth, Blue shrimp, Litopenaeus stylirostris, Dicentrarchus labrax, Milkfish, Chanos-chanos Forsskal
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






