 Research Article
Research Article
Exploring Indicators of Food Choice for Chimpanzees at Taï National Park, Côte d’Ivoire: Aroma and Antioxidants
Sebastian EW Opitz5, Antoine KN Guessan1,2, Georgette Konan1,3, Samo Smrke5; Alexia Glöss5, Barbara Schönbächler5, Babette Klopprogge5, Constant Ahoua1,4 and Chahan Yeretzian5*
1Centre Suisse de Recherches Scientifiques (CSRS) en Côte d’Ivoire
2University Jean Lorougnon Guédé, Daloa, Côte d’Ivoire
3University Félix Houphouet Boigny, Abidjan, Côte d’Ivoire
4University Nangui Abrogoua, Abidjan, Côte d’Ivoire
5Zurich University of Applied Sciences (ZHAW), Institute of Chemistry and Biotechnology, Einsiedlerstrasse 31, 8820 Wädenswil; Switzerland
Chahan Yeretzian, Zurich University of Applied Sciences (ZHAW), Institute of Chemistry and Biotechnology, Einsiedlerstrasse 31, 8820 Wädenswil, Switzerland.
Received Date: January 26, 2020; Published Date: February 18, 2020
Abstract
This study explored the food choice for chimpanzees at Taï National Park, Côte d’Ivoire and attempted to link these to the aroma and antioxidants content. The focus was on the aroma composition and antioxidant content of the fruits of the tree Sacoglottis gabonensis (Baill.) Urb., S. gabonensis, at different ripeness stages, in order to elucidate potential drivers for the selection of presumably ripe fruits by the apes. Flavour analyses have revealed clear differences between the unripe, ripe and to a lesser extent also between ripe and overripe fruits. Eating fruits at a ripe stage implies that chimpanzees are rewarded with high level of macro-nutrients, e.g. carbohydrates together with low content of unwanted bitter compounds. Yet, ripe fruits do not represent a ripeness stage with highest antioxidant content and highest antioxidant content was found in unripe fruit, which is gradually decreasing while ripening.
Introduction
Taï National Park in the southwest of Côte d’Ivoire is the largest remaining tropical rain forest in West Africa and covers 555,000 ha. While it is recognized as a “Biodiversity Hotspot”, with a rich natural flora and fauna, it is also one of the last remaining habitats of many endangered species. The Taï Forest reserve was created in 1926 and promoted to National Park status in 1972. It was recognized as a UNESCO Biosphere Reserve in 1978 and added to the list of Natural World Heritage Sites in 1982. Among the many endangered species living in the Taï National park, one species of particularly concern is the chimpanzee (Pan troglodytes verus Blumenbach 1779), a member of the great ape family. Chimpanzees have already disappeared from four African countries, and are nearing extinction in many others, such as the Côte d’Ivoire where a survey reveals a sharp decline of 90 % (from 8 000-12 000 individuals in 1990 to 800-1200 in 2007) [1]. In the Taï National Park, the situation is currently stable with an estimated chimpanzee population of 480 individuals [2].
Primates living in such natural habitats face various constraints for their nutritional needs. Chimpanzees are regarded ripe fruit specialists [3]. Eating predominantly ripe fruits, chimpanzees obtain a higher dietary quality compared to other frugivorous monkeys, whereas during fruit scarcity also other plant parts are consumed [3]. Besides plant food, also vertebrates and invertebrates are part of their diet [4]. Chimpanzees are able to manage environmental constraints, such as e.g. seasonality of food availability. By adapting their feeding behaviour [5,6], they are able to take nutritional advantage of the temporal abundance of ripe fruits to reach a high supply of carbohydrate in their diet [4-7]. Similarly, they consume the fruit flesh (pulpa) of Sacoglottis gabonensis (Baill.) Urb. (Malpighiales: Humiriaceae) as well as the hard seed by using tools [8], again taking full advantage of the nutritional content of the fresh fruits.
Yet, they still have to deal with structural and chemical aspects of the available plants and fruits. As argued by Janzen [8], flora is not just green, but is colored by compounds such as morphine, caffeine, tannin or terpene. Particularly for fruits, chemical components and physical characteristics are often designed either to repulse or attract animals (or humans), with the objective to favour dispersion of the species. Impact of secondary plant metabolites and fruit colour on food choice are well documented in birds and mammals [9-13]. While colour is qualified as an honest signal of food quality and macronutrient rewards for birds [14,15], it might not be enough for a decisive answer on the maturity stage of the fruit. Therefore, primates were observed to use, in addition to colour, different sensory cues, such as the firmness (haptic) by biting and the smell (volatile aroma compounds) by sniffing the fruits, in order to judge the level of ripeness of a fruit. Hence, the aroma of fruits may be an important indicator of food quality, proving useful information to the animals about availability and presence of beneficial nutrients. Especially for nocturnal monkeys olfactory guided foraging plays an important role at narrow range as visual cues cannot be exploited [16].
In this study, it was observed that chimpanzees from the Taï National Park smell on the fruits of the tree Sacoglottis gabonensis (Baill.) Urb., before deciding whether to eat or reject the fruit (N’Guessan, personal observation). Hence, in this study, we aim at examining the aroma composition of S. gabonensis fruits at different ripeness stages, in order to elucidate aroma compounds that may drive the selection of presumably ripe fruits by the apes. In addition, the antioxidant content of the fruits was measured to assess whether fruits, preferentially selected by the apes, were also characterized by a high antioxidant content.
Methods
Study site and fruit collection
The study was conducted at the Taï National Park in the southwest of Côte d’Ivoire with the aim of identifying clues for food choices of the apes. Researchers have conducted studies on chimpanzees’ communities, fully habituated to the presence of human observers since 1984. It is known that Chimpanzees never consume Sacoglotis fruits in trees. After selection and collection of fruits on the ground, they put several fruits in their mouth, mash and eject the stone.
During a field trip in September 2013 (by three authors of this paper: N’Guessan, Ahoua & Yeretzian), Sacoglottis gabonensis fruits have been collected in the natural habitat of the chimpanzees. Fruits were collected at the ground below the trees and the ripeness stages were judged by their texture, colour and smell. Fruits were separated in three lots of ripeness: unripe, ripe and overripe fruits. They were immediately processed by separating the flesh from the stone and immersing the flesh in liquid nitrogen (each in a small and labelled plastic bag) for storage in the Côte d’Ivoire. Later, for transport from the Côte d’Ivoire to Switzerland, fruits were transferred into dry ice.
Aroma profile of Sacoglottis gabonensis fruits measured with HS GC-MS
After arrival in Switzerland, the fruits were stored at -20 °C. For sample preparation, fruits were directly taken out of the freezer, cut into small pieces and immersed in liquid nitrogen for two minutes. Approximately twenty g of fruit was then homogenized in a ball mill (MM400, Retsch, Haan, Germany). Five g of fruit slurry was put into a headspace vial and stored in the fridge for less than 60 minutes until analysis by headspace gas chromatography coupled to mass spectrometry (HS-GC/MS). GC/MS analyses was performed on a 7890/5975N instrument (Agilent Technologies, Santa Clara, USA) equipped with a DB-WAX column (30m × 0.25mm ID, Agilent Technologies, Santa Clara, USA) in electron impact ionization mode. For the headspace equipment (Gerstel, Mühlheim an der Ruhr, Germany) a 2.5 mL headspace syringe with a syringe temperature of 55 °C was used with a flush time of 60 s. The incubation time of the sample was 10 min at 50°C while agitating at 250 rpm. The injection volume was 1 mL injected with an injection speed of 200.00 μL/s, a split of 5:1 and a helium flow of 1 mL/min. The GC run started at 35 °C for 5 min and was then heating with a ramp of 20°C/min to 240°C with a 5 min hold. For data analysis, the software MSD Chemstation (Version G1701 EA E.02.00.493, Agilent Technologies, Santa Clara, USA.) and a mass spectral library (NIST08, National Institute of Standards and Technology 2008) were used. Compounds were identified by comparison of MS spectra and retention times with the mentioned database. The volatile concentration in the headspace of the three ripeness stages was statistically analyzed using Kruskal-Wallis rank sum test, followed by a post-hoc test. For further differentiation between the samples, we performed a principal component analysis (PCA) on the HS GC/MS data, using the software package R (http://cran.rproject. org/, Tinn-R editor version 2.4.1.5, http://sourceforge.net/ projects/tinn-r/). Odour descriptors were taken from Flavornet by Terry Acree & Heinrich Arn (http://www.flavornet.org, © Datu Inc., 2004) and from The Good Scents Company™ (http://www. thegoodscentscompany.com).
Antioxidant capacity of Sacoglottis gabonensis fruits
For the antioxidant measurements, 500 mg of fruit slurry (see preparation for headspace analyses) were extracted three times with 10 mL of 70% aceton / 30% water phase. The extraction process included 10 minutes treatment in the ultrasonic bath and 2 min of mixing in a vortex. After evaporation, the residue was solved in 25 mL of water and filtered before analysis using 0.45 μm PET filters (Machery-Nagel, Düren, Germany). The Folin Ciocalteu (FC) reagent assay is measured on a FIAlab-3200 instrument (FIAlab Instruments Inc., U.S.A.) applying a FIA method [17]. The sample (diluted fruit extract) or antioxidant standard (gallic acid) were injected (injection loop 100 μl) into the flow stream (flow rate 30 μl/sec) of the FC reagent (0.2 M concentration). After mixing with sodium hydroxide (0.25 M concentration, flow rate 30 μL/ sec), to raise the solution pH for higher reactivity, dispersion in the reaction coil (1m tubing length) led to a mixing of the components and the reaction product (blue colored metal complexes) was measured photometrically (λ = 765 nm, slit 10 nm). A calibration curve was produced by analysis of gallic acid (GA) standards (gallic acid monohydrate, purity > 99 %, Sigma-Aldrich, SZBB0130V) at 765 nm. A stock solution was prepared by dissolving 50 mg GA in 100 mL degassed water and diluting with degassed water to provide working standard solutions of 10, 20, 30, 40, 50 and 60 ppm. For comparison with other studies, all results were related to the antioxidant activity of gallic acid and presented as gallic acid equivalent (GAE).
Results and Discussion
Aroma profiles of Sacoglottis gabonensis fruits
Chimpanzees were observed to actively sniff on S. gabonensis fruits prior to eating. Therefore, there have to be volatile cues emitted from the intact fruit that help the apes to judge e.g. its sensory quality and/or ripeness stage. Our analysis was performed on fruit slurry to increase intensities, which possibly takes also into account volatiles that might not be released and perceived from intact fruits (intact exocarp). Further, the protocol used here for sampling and storage of the fruit did only allow analyses of smashed fruits. However, among the detected substances were also typical fruit flavours, which would also be perceived through the intact exocarp.
Analyzing unripe, ripe and overripe fruits 22 volatile organic compounds (VOCs) were identified in the headspace of the smashed fruit flesh and chosen for characterization of the fruit aroma and for comparison of three maturity stages (Table 1). Only the absolute values of the HS-GC/MS headspace signal intensities from the 22 compounds can be presented here since quantification of headspace volatiles is difficult due to unknown partition coefficients of the volatiles between fruit matrix and headspace. Taking this into account, we applied the highest significance level (p<0.001; 99.9 %) for the decision on flavour differences between the three ripeness stages. In terms of hypothesis testing (Ho: there is no difference between the aroma at different ripeness levels) we strongly reduce the risk for Type I error. Regarding odour evaluation, we use descriptions made by humans. To the best of our knowledge, no such information is available for great apes.
Table 1: Compounds detected with HS-GC/MS in fruits of S. gabonensis at different ripeness stages and their flavor descriptors are presented. Given are mean values of absolute headspace intensities and their standard deviation (SD). Statistical analysis was done with Kruskal Wallis rank sum test. Statistical differences are denoted by small letters after analysis with a post hoc test (p<0.001). The sources for the flavour description are taken from AFlavornet by Terry Acree & Heinrich Arn (http://www.flavornet.org, © Datu Inc., 2004) and BThe Good Scents Company™. (http://www. thegoodscentscompany.com). ***flavour with high odour strength, ** flavor with medium odour strength, description taken from BThe Good Scents Company™.
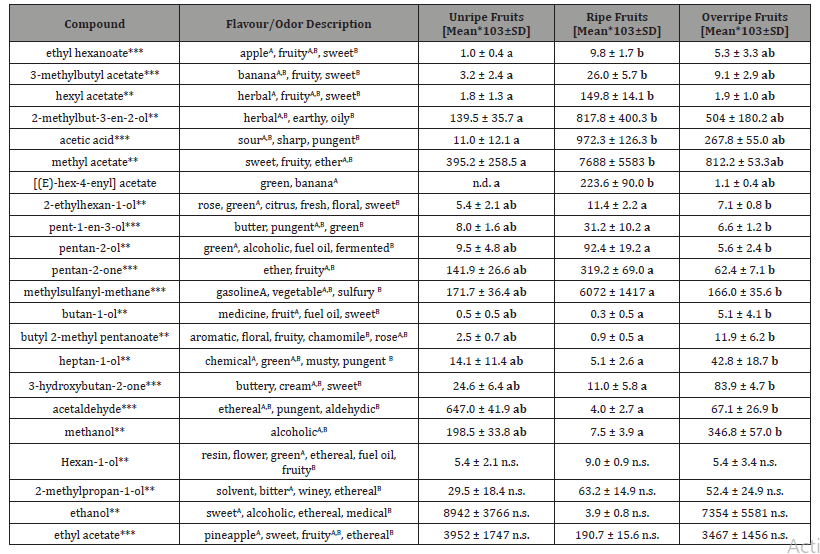
The values are plotted as the average of three fruits, measured each three times (Figures 1&2). The first set of compounds showed differences in concentrations between ripe and unripe fruits (Figure 1), while no significant difference to overripe fruits was detected. In a second set of compounds, the concentrations in ripe and overripe fruits (Figure 2) were differing, while no difference between ripe and unripe fruits was found.
intensities in ripe fruits (Figure 1), therefore bearing quantitative information to distinguish ripe fruits from unripe fruits. Fruity esters such as 3-methylbutyl acetate and ethyl hexanoate with high odour strength as well as hexyl acetate and methyl acetate with medium odour strength and [(E)-hex-4-enyl] acetate were more intense in ripe fruits (Table 1). The predominance of esters in ripe fruit is also described for other fruits [18].
Further, acetic acid with a sour and 2-methylbut-3-en-2-ol with an herbal flavour were also higher concentrated in ripe fruits. The fruity flavour methyl acetate was one of two dominant compounds in the headspace of S. gabonensis fruits, which should result in a fruity smell that likely can be perceived by chimpanzee. Therefore, ripe fruits should be well distinguishable from unripe fruits by a series of fruity flavours, which would help the apes in their decision to eat ripe fruits.
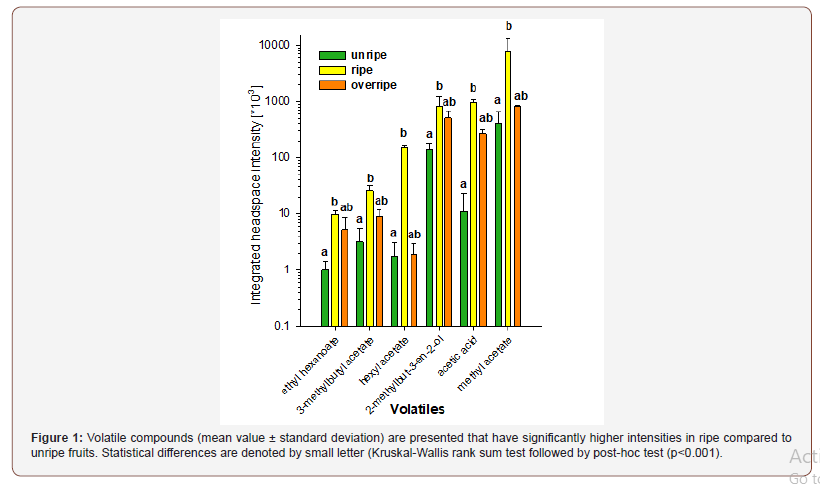
However, we were unable to collect fruits of different ripeness stages directly from the tree, leaving uncertainty on the exact ripeness degree of the collected fruits and how possible fermentation processes ongoing on the ground were influencing the aroma profile of ripe and overripe fruits.
When comparing ripe to overripe fruits, the differences in volatiles are less pronounced (Figure 2). Yet, a few points are important to be noticed. While several volatile compounds were present at significantly higher concentrations in ripe compared to overripe fruits, other compounds with similar odour descriptions were present at lower concentrations in ripe fruits. This was the case for fruity compounds, where pentan-2-one was more abundant in ripe fruits, whereas butyl 2-methylpentanoate, also known as a fruity compound, was ten times lower in ripe versus overripe fruits. Both volatile compounds were also present in unripe fruits, yet in concentrations that were not statistically different from either ripe or overripe fruits. A similar situation can be found for compounds commonly referred to having a “fusel oil” odour note. Pentan- 1-ol showed significantly higher intensities in ripe, whereas the situation was reversed for butan-1-ol (higher intensities in overripe fruits). Compounds being perceived as green or fresh, such as pent- 1-en-3-ol and 2-ethylhexan-1-ol, appeared in higher concentrations in ripe fruits, while heptan-1-ol was more abundant in overripe fruits. Particularly interesting are the alcoholic, pungent and fermented compounds methanol and acetaldehyde. Acetaldehyde and methanol are two well know toxic volatile compounds, that indicate the onset fermentation [19]. The EU regulation 1576/89 fixed common analytical composition limits for those volatiles (1000 g/hL AA for methanol and 73–500 g/hl AA for acetaldehyde). Found in higher concentration in overripe fruits, they indicate the onset fermentation. Ethanol as well as acetaldehyde, both having an ethereal odour, were both lowest in ripe fruits, yet not significantly lower than in unripe or overripe fruits (Table 1).
Acetaldehyde in regulating the fruit ripening (so minimum at ripe) [20].
While fermentation processes could explain the high content of ethanol in overripe fruits, the high content in unripe fruits is unexpected. As headspace analysis limited us to the analysis of pre-cut fruit flesh, besides highly volatile compounds also ethanol and methanol contained within the fruits was analyzed with our method. Under ideal conditions one would need to only probe the intact fruit, in order to resemble the situation of chimpanzees, where only volatiles that are emitted thorough the skin are relevant compounds for the decision on food choice.
Considering absolute intensities of headspace volatiles, the most abundant flavours in ripe fruits were identified as methylsulfanylmethane and methyl acetate. Besides the fruity flavour methyl acetate, methylsulfanylmethane is described as vegetable or sulfury. This compound with high odour strength might therefore also be a perceptible clue for chimpanzees to distinguish between ripe and unripe or overripe fruits. On the other hand, dominating flavours in unripe and ripe fruits were ethanol and ethyl acetate, both reaching several million counts in both ripeness stages. While both compounds are described to have an ethereal and sweet flavour, their high content is unexpected in unripe fruits. However, the high absolute intensities of ethanol and ethyl acetate in the headspace as well as 2-methylpropan-1-ol and hexan-1-ol were not statistically different between the three maturity stages.
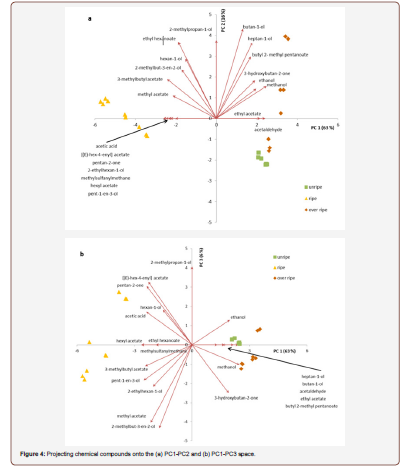
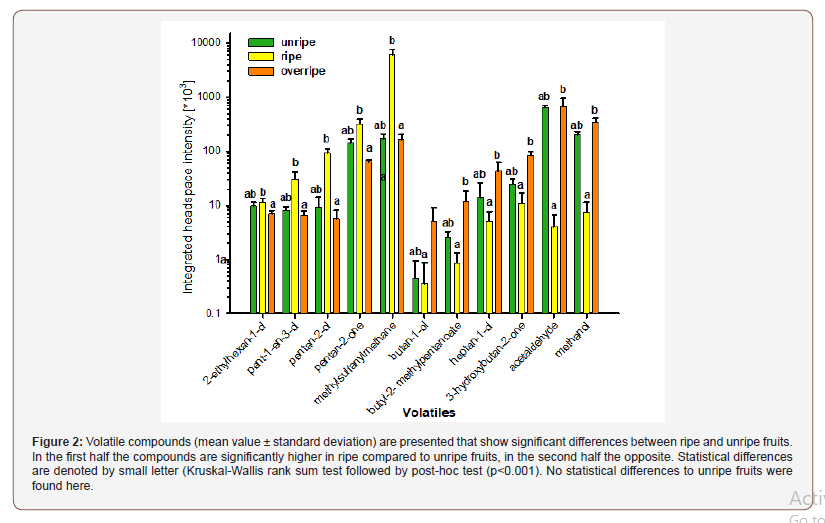
The three maturity stages were also compared by performing a principal component analysis. The PCA of the headspace intensities from three maturity stages of S. gabonensis fruits are presented in Figure 3 and Figure 4. Within the 3D-space of the three first principal components (Figure 3), the three types of fruits are clearly separated and distinguishable, based on the intensity profiles of all 22 selected aroma compounds. In Figure 4, the chemical compounds responsible for this separation are plotted in the 2D space of the respective principal components. Hexyl acetate, [(E)-hex-4-enyl] acetate, acetic acid, methylsulfanylmethane, 2-ethyl hexan-1-ol, pentan-2-one and pent-1-en-3-ol as well as methyl acetate are positively linked to ripe fruits as the loadings of these compounds (depicted by arrows) are directed towards the group of ripe fruits on the PC1-PC2 space (Figure 4a). These compounds showed also their highest intensities in ripe fruits. Compounds projecting towards unripe fruits were ethylacetate and acetaldehyde, ethanol and methanol towards overripe fruits. Similarly, when projecting the chemical compounds onto the PC1-PC3 space (Figure 4b), we see that acetaldehyde, ethyl acetate, heptan-1-ol, butan-1-ol, and butyl-2-methyl pentanoate as well as ethanol and methanol are negatively correlated to ripe fruits and are hence significantly less abundant in the ripe fruits than in unripe and/or overripe fruits.
Antioxidant capacity of Sacoglottis gabonensis fruits
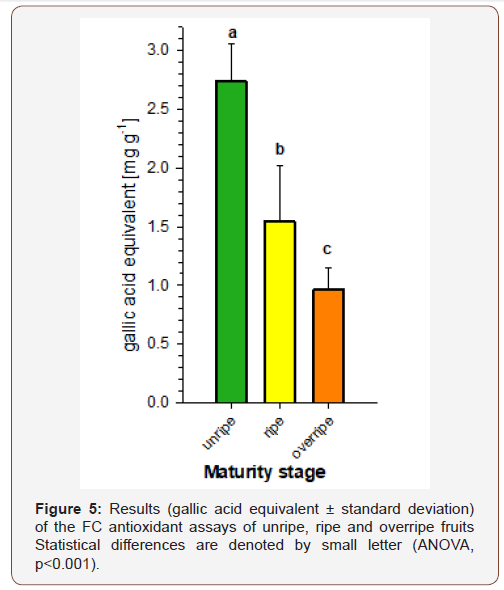
The three ripeness stages were analyzed with the Folin- Ciocalteu antioxidant assay, in order to investigate potential influence of health-related antioxidant level in fruits on the food choice of chimpanzees. After statistical analysis with ANOVA, the highest signal was produced with the unripe fruits showing the highest antioxidant activity, which was decreasing with ripeness (Figure 3). The unripe fruits showed a signal of 2.74 mg/g ± 0.31 mg/g (mean value ± standard deviation) gallic acid equivalent, while this value decreased for ripe fruits to 1.55 ± 0.48 mg/g GAE and was the lowest for overripe fruits with values at 0.97 ± 0.18 mg/g GAE (ANOVA: F = 41.09, df = 15, p = < 0.001). As presumably ripe fruits are chosen by the apes, the highest antioxidant capacity of the fruits is of minor priority in the food choice. The observed gradual decrease of antioxidant capacity during ripening is in accordance with earlier reports on other fruits, where the highest values are reported for unripe stages, while they degrade during ripening [21]. Nevertheless, also beneficial compounds are still present in ripening fruits, e.g. flavonoids, carotenoids, which are responsible for the color of the fruit.
Conclusion
Flavour analyses have revealed clear differences between the unripe, ripe and to a lesser extent also between ripe and overripe fruits. Ripe fruits are characterized by fruity esters like 3-methylbutyl acetate, methyl acetate, hexyl acetate, [(E)-hex-4- enyl] acetate and ethyl hexanoate. In contrast, simple aliphatic alcohols such as methanol, ethanol, butan-1-ol and heptan-1-ol are characteristic for unripe and overripe fruits, while comparably low intensities are present in ripe fruits. The first group of compounds can therefore be considered as “positive” markers for ripe fruits, which likely are also responsible for the food choice of chimpanzees, while the second group are “negative markers” that would help apes in rejecting unripe as well as overripe fruits. These fruity esters could be qualitative indicators for chimpanzees that hold information about the ripeness stage of the fruits. It is well documented that chimpanzees are able to distinguish between different ripeness stages [3,22-31]. On the other hand, flavours such as ethanol that are responsible for fermentation, could be indicator for rejecting overripe fruits.
Eating fruits at a ripe stage implies that chimpanzees are rewarded with high level of macro-nutrients, e.g. carbohydrates together with low content of unwanted bitter compounds, such as antifeedants [3]. Ripe fruits do not represent a ripeness stage with highest antioxidant content and highest antioxidant content was found in unripe fruit, which is gradually decreasing while ripening.
Acknowledgement
We would like to acknowledge the Swiss Center for Scientific Research in Côte d’Ivoire (CSRS) for its financial and logistical supports during data collection on the field through the UNPD 2 program. We are also grateful to the Ivorian Parks and Reserves Office (OIPR) who allowed us to access the Taï National Park for fruits samples collection.
Conflict of Interest
No conflict of interest.
References
- Campbell G, Kuehl H, N Goran KP, Boesch C (2008) Alarming decline of West African chimpanzees in Côte d'Ivoire. Curr Biol 18
(19): R903-R904. - N’ Goran KP, Yapi AF, Normand E, Herbinger I, Diarrassouba A, et al. (2011) Etat du Parc National de Taï: Rapport de résultats de biomonitoring phase VI, Abidjan.
- Wrangham RW, Conklin Brittain NL, Hunt KD (1998) Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. I. Antifeedants. International Journal of Primatology 19: 949-970.
- Chapman CA, Fedigen LM (1990) Dietary Differences between Neighboring Cebus-Capucinus Groups - Local Traditions, Food Availability or Responses to Food Profitability. Folia Primatol (Basel) 54(3-4): 177-186.
- Dunbar RIM (1988) Primate social systems, Comstock Pub, Cornell University Press, USA.
- N guessan AK, Ortmann S, Boesch C (2009) Daily Energy Balance and Protein Gain Among Pan troglodytes verus in the Taï National Park, Côte d Ivoire. International Journal of Primatology 30: 481-496.
- Wrangham R, Clark AP, Isabirye Basuta G (1992) Female social relationship and social organisation of Kibale Forest chimpanzees. In Nishida T, Mcgrew WC, Marler P, Pickford M, Waal FBMD (eds.) Topics in Primatology, University of Tokyo Press, Japan.
- Janzen DH (1978) Complications in interpreting the chemical defenses of trees against tropical arboreal plant-eating vertebrates. In Montgomery GG (eds.) Ecology of the arboreal florivores. Washington DC, Smithsonian Institution Press, USA.
- Ganzhorn JU (1986) Food Selection of Lemur Catta and Lemur Fulvus. International Journal of Primatology 5: 340-340.
- Janzen DH, Juster HB, Bell EA (1977) Toxicity of Secondary Compounds to Seed-Eating Larvae of Bruchid Beetle Callosobruchus-Maculatus. Phytochemistry 16: 223-227.
- Schaefer HM, Schaefer V, Levey DJ (2008) How plant-animal interactions signal new insights in communication. Trends in Ecology & Evolution 19(11): 577-584.
- Wheelwright NT, Janson CH (1985) Colors of Fruit Displays of Bird-Dispersed Plants in Two Tropical Forests. The American Naturalist 126: 777-799.
- Willis AJ, Mcewan JWT, Greenwood JJD, Elton RA (1980) Food Selection by Chicks-Effects of Color, Density, and Frequency of Food Types. Animal Behaviour 28: 874-879.
- Schaefer HM, Mcgraw K, Catoni C (2008) Birds use fruit colour as honest signal of dietary antioxidant rewards. Functional Ecology 22(2): 303-310.
- Schaefer HM, Schmidt V (2004) Detectability and content as opposing signal characteristics in fruits. Proc Biol Sci 271: S370-S373.
- Bolen RH, Green SM (1997) Use of olfactory cues in foraging by owl monkeys (Aotus nancymai) and capuchin monkeys (Cebus apella). J Comp Psychol 111(2): 152-158.
- Goodall J (1986) The chimpanzees of Gombe: patterns of behavior, Cambridge, Mass, Belknap Press of Harvard University Press, USA.
- Newton Fisher, NE (1999) The diet of chimpanzees in the Budongo Forest Reserve, Uganda. African Journal of Ecology 37(3): 344-354.
- Nishida T (1990) The Chimpanzees of the Mahale mountains: sexual and life history strategies, University of Tokyo Press, Japan.
- Kormos R, Boesch C, Bakarr MI, Butynski TM (2004) Chimpanzés d Afrique de l' Etat de conservation de l espèce et plan d action, Groupe de spécialistes des primates de la CSE de l UICN, Gland, Suisse et Cambridge, Royaume-Uni.
-
Chahan Yeretzian, Sebastian EW Opitz, Antoine KN Guessan, Georgette Konan, Samo Smrke, et al. Exploring Indicators of Food Choice for Chimpanzees at Taï National Park, Côte d’Ivoire: Aroma and Antioxidants. World J Agri & Soil Sci. 4(2): 2020. WJASS.MS.ID.000588.
-
Chimpanzees, Taï National Park, Aroma, Antioxidants, S. gabonensis, Sacoglotis fruits, Mass spectrometry
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






