 Research Article
Research Article
Secondary Metabolites Profiling, ProximateCompositions and Antimicrobial Activities of CordyliaPinnata
Alawode Rahmatallah Adenike1*, Dauda Muhammed1, Adegbola Gbolagade Adeyemi1 and BabatundeRichard O2
1Forestry Research Institute of Nigeria -Southern Guinea Research Station Mokwa, Nigeria
2Federal College of Forestry, Ibadan, Nigeria
Alawode Rahmatallah Adenike, Forestry research instituteof Nigeria Southern Guinea Research station Mokwa, Nigeria.
Received Date: September 23, 2019; Published Date: October 01, 2019
Abstract
Background: This study was carried out to investigate proximate composition, phytochemical profile and antimicrobial activity of the aqueousextract of Cordylia pinnata.
Methods: Phytochemical and proximate composition was determined using standard procedures. Antimicrobial test against S. aureus, E. coli,and S. typhi was investigated using agar diffusion method.
Results: Cordylia pinnata contains phenol, flavonoids, tannins, alkaloids, saponins and glycosides. Quantitatively, Cordylia pinnata containsalkaloids (25.05±0.04 mg/100g), flavonoids (26.12±0.78 mg/100g), cyanide (8638.80±15.67 mg/100g), phenols (156.89±0.67 mg/100g),saponins (1564.10±0.94 mg/100g), tannins (31.59±0.83 mg/100g). The proximate compositions show that Cordylia pinnata contains moisture(3.46±0.32%), ash (7.42±0.11 %), proteins (13.13±0.21 %), fibers (13.5±0.12 %), Carbohydrate (57.57±0.34 %). The extract was more sensitive toSalmonella typhi with inhibition zone in the range from 23.00 ±0.67 mm to 30.00±0.67mm. Zone of inhibition of E. coli ranged from 18.50±0.04mmand 27.00±0.56mm. The extract was sensitive to Staphylococcus aureus only at the concentrations of 80mg/mL (20.00±0.35mm) and 100mg/mL(26.00±0.89mm).
Conclusion: Aqueous extract of Cordylia pinnata contains significant amounts of nutrients which if consumed in sufficient amount couldcontribute greatly towards meeting human nutritional requirement for normal growth and adequate protection against diseases arising frommalnutrition. The extract contains appreciable amounts of phytochemicals which might have contributed to its antimicrobial activities.
Keywords:Cordylia pinnata; Phytochemicals; Proximate; Antimicrobial
Introduction
Infectious diseases are responsible for an overwhelmingnumber of deaths and morbidity worldwide [1]. In tropical regionsof the world, in particular, developing countries like Nigeria, poorhealth is prevalent and diseases such as malaria, meningitis,pneumonia, tuberculosis and gastrointestinal infections stronglypersist. From ancient times, different parts of medicinal plants havebeen used to cure specific ailments [2].
Recently, a gradual revival of interest in the use of medicinalplants in developing countries was rekindled because herbalmedicines have been reported to be safe and without any adverseside effect especially when compared with synthetic drugs [3].They are still actively used against some infections as primary carebefore seeking conventional treatment at hospitals [2].
Medicinal herbs are a source of chemical compounds such asalkaloids, glycosides, saponin, oleoresins, sesqueterpine, lactonesand oils [4]. These biologically active ingredients are used for theprophylactic purposes and for the different infectious diseases [5,6].Due to the presence of medicative properties, medicinal plants havebeen used in wide area of the world. Many diseases like malaria,trypanosomiasis, oxidative stress, epilepsy, diarrhea, dysentery,fungal and bacterial infections have been treated by folkloremedicines and have received substantial scientific validation [7-11].
Cordyla pinnata Lepr. ex A. Rich. commonly known as Bushmango. It’s a flowering plant belonging to Fabaceae family. It isnative to Western Tropical Africa from Senegal to Nigeria. The plantis harvested from the wild and used locally to supply wood, foodand medicine [11]. In African traditional medicine, the leaves, rootand bark decoctions of Cordyla pinnata are used as tonic, waterpurifier, diuretic, oxytocic, cholagogue, aphrodisiac, and to treat,diarrhoea, gastro-intestinal discomfort, worms, lumbago, syphilisand schistosomiasis [12].
Due to the paucity of information on phytochemistry andproximate composition of this plant, this study was necessitatedto investigate the proximate compositions, antimicrobial activityand bioactive compounds in the leaf of Cordyla pinnata whichmay provide baseline information for their subsequent utilizationin supplementing nutritional requirements and for use asantimicrobial herb.
Materials and Methods
Sample collection and extraction
Fresh leaves sample of Cordylia pinnata was collected in March2019 at Mokwa Nigeria. The leaves were thoroughly washed underrunning tap water to remove all contaminants after which theywere cut into pieces, dried for 2 wk (37oC) and finally groundedusing a grinder mill. A 50 g of the plant material was maceratedwith 300 mL of distilled water for 24 hrs and the resulting extractwas filtered and concentrated using water bath.
Qualitative and Quantitative screening for secondarymetabolites
The plant extract was analyzed for the presence of somesecondary metabolite including alkaloids, terpenes, tannins,saponins, phenols, steroids, phlobatannins and flavonoids usingstandard procedures [13-15]. Quantitative analysis was conductedfor flavonoid, alkaloids, total phenol, tannin and saponins usingstandard procedures [16].
Proximate analysis
The proximate compositions of Cordylia pinnata leaf including;crude proteins, crude fiber, moisture content, ash content, crudefat and carbohydrate were determined using standard procedures[16].
Antimicrobial activity
The antimicrobial activities of the fractions were evaluated atconcentrations of 20, 30, and 40 mg/ml using agar well diffusionmethod as described by Yusuf, et al. [17] and Tsado, et al. [18]. Theantibacterial activity was expressed as the mean zone of inhibitiondiameters (mm) produced by the plant fractions.
Statistical analysis
Values were analyzed using statistical package for socialscience (SPSS) version 21 and presented as means ± SE of the mean.Comparisons between different groups were carried out by onewayanalysis of variance (ANOVA) followed by Duncan’s MultipleRange Test (DMRT). The level of significance was set at P < 0.05.
Conclusion and Recommendations
" class="head-link">Conclusion and Recommendations
Qualitative phytochemicals
The qualitative phytochemical compositions of Cordylia pinnataare presented in Table 1. Cordylia pinnata contains Total phenol,total flavonoids, tannins, alkaloids, saponins and glycosides.Anthraquinone was however not present.
Quantitative phytochemicals
The quantitative phytochemical compositions of Cordyliapinnata are presented in Table 2. Cordylia pinnata contains alkaloids(25.05±0.04 mg/100g), flavonoids (26.12±0.78 mg/100g), cyanide(8638.80±15.67 mg/100g), phenols (156.89±0.67 mg/100g),saponins (1564.10±0.94 mg/100g), tannins (31.59±0.83 mg/100g).
Proximate composition
The proximate compositions of Cordylia pinnata is presentedin Table 3. Cordylia pinnata contains moisture (3.46±0.32%), ash(7.42±0.11 %), proteins (13.13±0.21 %), fibers (13.5±0.12 %),Carbohydrate (57.57±0.34 %).
Antimicrobial activities
The Zones of inhibition of Salmonella typhi, Staphylococcusaureus and E. coli by aqueous extract of Cordylia pinnata ispresented in Table 4. The zones of inhibition of Salmonella typhi,Staphylococcus aureus and E. coli demonstrated by the extractincreased with increasing concentration. The extract was moresensitive to Salmonella typhi with inhibition zone in the rangefrom 23.00 ±0.67 mm to 30.00±0.67 mm. zone of inhibition of E.coli ranged from 18.50±0.04 mm and 27.00±0.56 mm. The extractwas sensitive to Staphylococcus aureus only at the concentrationsof 80 mg/mL (20.00±0.35 mm) and 100 mg/mL (26.00±0.89 mm)(Table 1-4).
Table 1: Qualitative phytochemical composition of Cordylia pinnata.
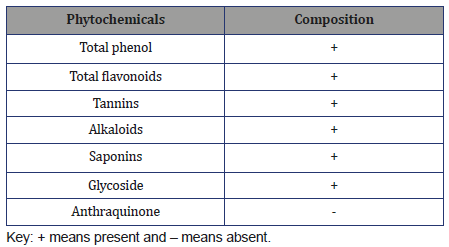
Table 2: Quantitative phytochemical composition of Cordylia pinnata.
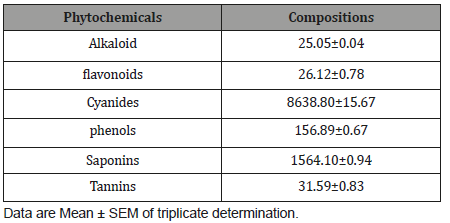
Table 3: Proximate composition of Cordylia pinnata.
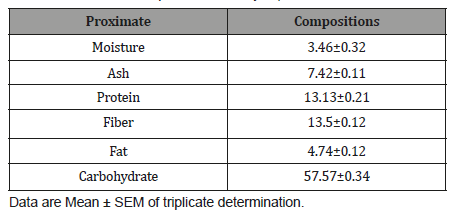
Table 4: Antimicrobial activities of crude aqueous leaf extract of Cordyliapinnata.
Discussion
The result of the phytochemical screening is as shown in Table1. These phytoconstituents are secondary metabolites, whichare biologically active and may be applied in nutrition and aspharmacologically active agents [19]. The presence of glycoside maysupport against cardiovascular disorder since cardiac glycosidesare clinically used in the treatment of congestive heart failure andas anti-arrhythmic agents due to their strong inhibitory activitytoward the ubiquitous cell surface enzyme Na+/K+-ATPase [20]. Inorder to formulate, produce and market quality diets it is necessaryto gain information about substance classes that contribute to theenergy content and special compounds, which influence digestibility[21]. Each medicinal plant species has its own nutrient compositionbesides having pharmacologically important phytochemicals.
Nutrients are essential for the physiological functions of humanbody. Such nutrients and biochemicals like carbohydrates, fatsand proteins play an important role in satisfying human needs forenergy and life processes [22]. Hence, the proximate analysis wasinvestigated in order to ascertain the nutritive importance of thesample for possible human consumption as well as in livestockfeed meal formulation. The moisture content was 3.46±0.32%,which allow for improved activity of watersoluble enzymes and coenzymesneeded for metabolic activities. Moisture content is amongthe most vital and mostly used measurement in the processing,preservation and storage of food [23]. Thus, this low moisturecontent implies that the product possess high shelf life. The drymatter component was 96.54±2.48%, which was the summed totalof ash (7.42±0.11 %), proteins (13.13±0.21 %), fibers (13.5±0.12%) and Carbohydrate (57.57±0.34 %). The protein content of theplant was found to be 13.13±0.21 %, which shows that the plantcan serve as a source of protein considering the level of proteindeficiency in the society today. Its intake can contribute to theformation of hormones, which controls a variety of body functionssuch as growth, repair and maintenance. The crude fibre was13.5±0.12 %. In the intestinal tract, fiber resists being broken downby enzymes; although, part of it may be metabolized by bacteria inthe lower gut [21]. Fiber is characterized by low or no nutritionalvalue, but because of its effect on the digestive system, it is thoughtto help with such problems as diabetes and high levels of bloodcholesterol [24, 25]. This high crude fiber might aid in combatinggastrointestinal disorder, since, individuals with high intakes ofdietary fiber appear to be at significantly lower risk for developingcoronary heart disease, stroke, hypertension, diabetes, obesityand certain gastrointestinal diseases [26]. The carbohydrate iscategorized as the nitrogen free extract (extract), which comprisesof the easily digestible carbohydrates such as sugar, starch andorganic acids [21]. The amount of carbohydrate herein was57.57±0.34 %, which conferred on the plant, significant roles tohuman health. This is because, apart from the supply of energy,carbohydrates are also needed in numerous biochemical reactionsnot directly concerned with energy metabolism as earlier reportedby Bhattacharjee et al. [27].
Antimicrobial sensitivity testing was carried out in vitrousing agar-well diffusion method against Salmonella typhi,Staphylococcus aureus and E. coli. The aqueous extract of Cordyliapinnata had varying degree of antimicrobial activity against the testorganisms. On the basis of the results the extract demonstrated tobe more active on E. coli and least active on Staphylococcus aureus.This was not a surprise since previous studies [28] have reportedthat generally plant extracts are usually more active againstGram-positive bacteria than Gram-negative bacteria, and thesusceptibility may be due to structural differences in the cell wallof these classes of bacteria [29]. Cells of Gram-negative bacteria aresurrounded by an additional outer membrane, which provide themwith a hydrophilic surface that functions as a permeability barrierfor many substances including natural compounds [30]. It was alsoobserved that increase in the concentration of all the extract yieldedtheir corresponding increase in the zones of inhibition. This linearrelationship between the concentrations of extracts and zones ofinhibition could be that the extracts were able to diffuse into theinoculated nutrient agar [31-33]. The ability of the extract toinhibit the growth Staphylococcus aureus and E. coli explains whyit is used in folk medicine for the treatment of infectious diseases.However, despite the higher zone of inhibition demonstratedby the plant extract the zone of inhibitions was lower on all testorganism compare to zone of inhibitions demonstrated by standardantibiotics drug (ciprofloxacin) used in this study.
Conclusion
Aqueous extract of Cordylia pinnata contains significantamounts of nutrients which if consumed in sufficient amount couldcontribute greatly towards meeting human nutritional requirementfor normal growth and adequate protection against diseases arisingfrom malnutrition. The extract contains appreciable amounts ofphytochemicals which might have contributed to its antimicrobialactivities.
Acknowledgment
The author would like to appreciate the technical staff ofDepartment of Biochemistry, and Department of Chemistry, FederalUniversity of Technology Minna for their kind assistance duringthe study. Appreciation also goes to Messrs Salihu Chiji and AliyuShaba of Southern Guinea Research Staton, Mokwa, for assistancerendered during collection of samples.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Madikizela B, Ndhlala AR, Finnie JF, Van Staden J (2012) Ethnopharmacological study of plants from Pondoland used against diarrhea. J Ethnopharmacol 141(1): 61-71.
- Mahato RB, Chaudhary RP (2005) Ethnomedicinal study and antibacterial activities of selected tropical plants. Revista Cubana de Plantas Medicinales 9: 1-6.
- Osunlana OR, Bello MO, Johnson JA (2018) Nutritive values and bioactive compounds content of three commonly used blood pressure regulating plant leaves. Archives 1: 128-136.
- Singh AP (2005) Promising Phytochemicals from Indian Medicinal Plants. Ethnobotanicals Leaflets 2005(1): Article18.
- Odangowei IO, Ngozi GE, Oluchi GD (2019) Phytochemical, proximate and mineral compositions of Bryophyllum Pinnatum (Never die) medicinal plant. Journal of Pharmacognosy and Phytochemistry 8(1): 629-635.
- Anpin Raja RD, Jeeva S, Prakash JW, Johnson M, Irudayaraj V (2011) Antibacterial Activity of Selected Ethnomedicinal Plants from South India. Asian Pac J Trop Med 4(5): 375-378.
- Lawal B, Shittu OK, Oibiokpa FI, Berinyuy EB, Muhammed H (2016) African natural products with potential antioxidants and hepatoprotectives properties, a review. Clinical Phytoscience 2(23): 1-66.
- Sofowora A (1996) Research on Medicinal Plants and Traditional Medicine in Africa. J Altern Complement Med 2(3): 365-372.
- Bashir L, Shittu OK, Sani S, Busari MB, Adeniyi KA (2015) African Natural Products with Potential Antitrypanosoma Properties, A Review. International Journal of Biochemistry Research & Review 7(2): 45-79.
- Lawal B, Shittu OK, Kabiru AY, Jigam AA, Umar MB, et al. (2015) Potential antimalarials from African natural products, A review. J Intercult Ethnopharmacology 4(4): 318-343.
- Kirkbride JH (2005) Dupuya, a new genus of Malagasy Legumes (Fabaceae). Novon 15(2): 305-314.
- Nyunaï N (2011) Cordyla pinnata (Lepr. ex A.Rich.) Milne-Redh. (Internet) Record from PROTA4U. Lemmens RHMJ , Louppe D, Oteng-Amoako AA (Eds.). PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Netherlands.
- Harborne JB (1993) Phytochemical Methods; A guild to modern Techniques to plant analysis. Freeman and Company pp. 78-80.
- Trease GE, Evans WC (1989) Pharmacognosy. 11th (edn), Brailliar Tiridel CanMacmillan Publishers, London pp. 60-75.
- Sofowora AE (1993) Recent trends in research into African medicinal plants. J Ethnopharmacol 38(2-3):209-214.
- AOAC (Association of official analytical chemist) (2000) Official Method analytical chemist, Washinton DC.
- Yusuf AA, Lawal B, Yusuf MA, Omonije YO, Adejoke AA, et al. (2018) Free Radical Scavenging Antimicrobial Activities and Effect of Sub-Acute Exposure to Nigerian Xylopia Aethiopica Seed Extract on Liver and Kidney Functional Indices of Albino Rat. Iranian journal of toxicology 12(3): 51-58.
- Tsado NA, Lawal B Kontagora GN, Muhammad BM, Yahaya MA, Hassan MK, et al. (2016) Antioxidants and Antimicrobial- Activities of Methanol Leaf Extract of Senna occidentalis. Journal of Advances in Medical and Pharmaceutical Sciences 8(2): 1-7.
- Kirtikar KR, BD Basu (2006) Indian Medicinal Plants. Bishan Singh Mahendra Pal Singh (Ed.), Dehra Dun, India, pp: 892-894.
- Shirwaikar A, V Parmar, S Khan (2011) The changing face of nutraceuticals-An overview. Int J Pharm Life Sci 2: 925-932.
- Soetan KO, OE Oyewole (2009) The need for adequate processing to reduce the antinutritional factors in plants used as human foods and animal feeds: A review. Afr J Food Sci 3(9): 223-232.
- Bhaskaram P (2001) Immunobiology of mild micronutrient deficiencies. Br J Nutr 85(2): S75-S80.
- Eme OI, T Onyishi, OA Uche, IB Uche (2014) Challenges of food security in Nigeria: Options before government. Arabian J Bus Manage Rev 4: 15-25.
- Iheanacho KME, AC Udebuani (2009) Nutritional composition of some leafy vegetables consumed in Imo State Nigeria. J Applied Sci Environ Manage 13(3): 35-38.
- Eastwood M, D Kritchevsky (2005) Dietary fiber: How did we get where we are? Annu Rev Nutr 25: 1-8.
- Anderson JW, P Baird, RH Davis Jr, S Ferreri, M Knudtson, et al. (2009) Health benefits of dietary fiber. Nutr Rev 67(4): 188-205.
- Bhattacharjee S, A Sultana, MH Sazzad, MA Islam, MM Ahtashom (2013) Analysis of the proximate composition and energy values of two varieties of onion (Allium cepa L.) bulbs of different origin: A comparative study. Int J Nutr Food Sci 2: 246-253.
- Hemaiswarya S, Kruthiventi AK, Doble M (2008) Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15(8): 639-652.
- Briers Y, Lavigne R (2015) Breaking barriers: expansion of the use of endolysins as novel antibacterials against Gram-negative bacteria. Future Microbiol 10(3): 377-390.
- Tsado AN, Lawal B, Ossai PC, Jagaba A, Gwadabe NK, et al. (2016) Antioxidants and Antimicrobial Activities of Methanol Extract of Newbouldia laevis and Crateva adansonii. Journal of Pharmacy and Allied Health Sciences 6: 14-19.
- Lawal B, Shittu OK, Oibiokpa IF, Mohammed H, Umar SI, et al. (2016) Antimicrobial evaluation, acute and sub- acute toxicity studies of Allium sativum. Journal of Acute Disease 5(4): 296-301.
- AM Ibrahim, B Lawal, AN Abubakar, NA Tsado, GN Kontagora, et al. (2017) Antimicrobial and Free Radical Scavenging Potentials of N-Hexane and Ethyl Acetate Fractions of Phyllanthus Fraternus. Nigerian Journal of Basic and Applied Science 25(2): 6-11.
- Yusuf AA, Lawal B, Abubakar AN, Berinyuy EB, Omonije YO, et al. (2018) In-vitro antioxidants antimicrobial and toxicological evaluation of Nigerian Zingiber officinale Clinical Phytoscience 4(12): 1-8.
-
Alawode Rahmatallah Adenike, Dauda Muhammed, Adegbola Gbolagade Adeyemi, Babatunde Richard O. Secondary MetabolitesProfiling, Proximate Compositions and Antimicrobial Activities of Cordylia pinnata. Sci J Biol & Life Sci. 1(1): 2019. SJBLS.MS.ID.000503.
Metabolites, Profiling, Proximate, Compositions, Antimicrobial, Cordylia pinnata, Phytochemicals, Morbidity, Worldwide, Alkaloids, Glycosides, Saponin, Oleoresins, Sesqueterpine, Lactones, Oils
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






