 Research Article
Research Article
Isolation and Characterization of Amylase Enzymefrom Selected Fungal Strains of Wof Washa Forest ofNorth Shoa, Ethiopia?
Mesfin Moges1, Negussie F Bussa2*, Manikandan Muthuswamy1 and Melese Abdisa3
1Department of Biology, Ethiopia
2Department of Food Science and Postharvest Technology, Ethiopia
3Science Faculty, Ethiopia
Negussie F Bussa, Department of Food Science andPostharvest Technology, Ethiopia.
Received Date: October 21, 2019; Published Date: November 21, 2019
Introduction
Among the major classes of microbial enzymes contributingimmediate application are the hydrolytic enzymes [1], whichaccount approximately 75% of the industrial enzymes produced.Through the use of hydrolytic enzymes many different naturaland agricultural polymers such as starch was processed and upgraded for eventual human or animal consumption, or for furtherbioconversion into value added products.
Starch degrading enzymes called amylases have received agreat deal of attention because of their perceived technologicalsignificance and economic benefits. Amylases have got increasingdemand and potential application in a wide number of industrialprocesses. This is because of the enormous interest in developingenzymes with better properties such as raw starch degradingamylases suitable for industrial applications and their cost-effectiveproduction techniques [2].
Amylase enzymes make use of starch and related oligo andpolysaccharides to cleave glycosidic bonds either from the nonreducingend (exo-acting enzymes) or in the interior (endo-actingenzymes) of the polymer. These enzymes have useful applicationsin the brewing, textile, paper, detergent, and pharmaceuticalindustries. They are also used in the production of maltose,oligosaccharide mixtures, and high fructose and maltotetraosesyrup industries [3]. Furthermore, the application of amylaseexpanded in many fields such as clinical, medicinal and analyticalchemistry [4]. The three best known amylases are α-amylase,β-amylase and glucoamylase that can be produced from fungi,yeasts, bacteria, plants and animals which are among the mostimportant enzymes in present day biotechnology [5].
The amylases produced by microorganisms have a broadspectrum of industrial applications as they are more stablethan plant and animal amylases. The major advantage of usingmicroorganisms for the production of amylases is the economicalbulk production capacity and the fact that microbes are easyto manipulate to obtain enzymes of desired characteristics [6].Microbial amylase can be produced by submerged as well as solidstate fermentations. Solid state fermentation holds tremendouspotentials for the production of amylase. It can be of special interestin those processes where the crude fermented product may beused directly as the source of amylase. This method has economicvalue for countries with abundance of biomass and agro industrialresidues, as these can be used as cheap raw materials.
However, the growth of microorganism and the resultedamylase are strongly influenced by medium composition. Thus,optimization of media components and cultural parameters is theprimary task in a biological process in order to obtain a good resultin amylase production. Therefore, to meet the growing demandsfor these enzymes in various industries, it is necessary to improvethe performance of the system so as to increase the yield withoutincreasing the cost of production especially in developing countrieslike Ethiopia [7].
In Ethiopia, most of its economy is dependent on agriculture,which makes it beneficiary from starch and starch products. In this regard, industries that utilize starch and other agriculturalraw materials to provide quality products use large quantities ofamylases which have a considerable economic significance. Forthis reason, the development of starch-based industries is crucialto address the demand of the current society. But this requiresthe presence of promising starch hydrolyzing amylase frommicroorganisms that enable to degrade a variety of agriculturalproducts efficiently [8].
In spite of the above facts, insufficient work was carried outin the production and characterization of fungal starch degradingamylases from environmental sources in Ethiopia. In order to fillsome of these gaps of research, the study was aimed and designedto isolate, screen and characterize amylase from soil inhabitingmicrobial sources, such as fungal strains from forest soils.
Materials and Methods
Description of the study area
The study was conducted at Addis Ababa University, ScienceFaculty Centre for Food Science and Nutrition Program. Sampleswere taken from Wof Washa Forest (WWF) located at 9°44′32″-9°46′26″N and 39°44′00″-39°47′19″E in North Shoa, Ethiopia. Thealtitude of the study area ranged from 1700 to 3910 m above sealevels and the temperature ranged from a mean annual minimumof 10° C at Kundi peak in Ankober to a mean annual maximum of20° C at lower altitudes. The forest area stretched from Southeastto Northwest along the rift valley escarpment on its eastern part. Itdivided the Awash and Nile basins on its western part and covers78 km2 Figure 1. The soils could be categorized based on theircolours as black, brown and red (Figure 1).
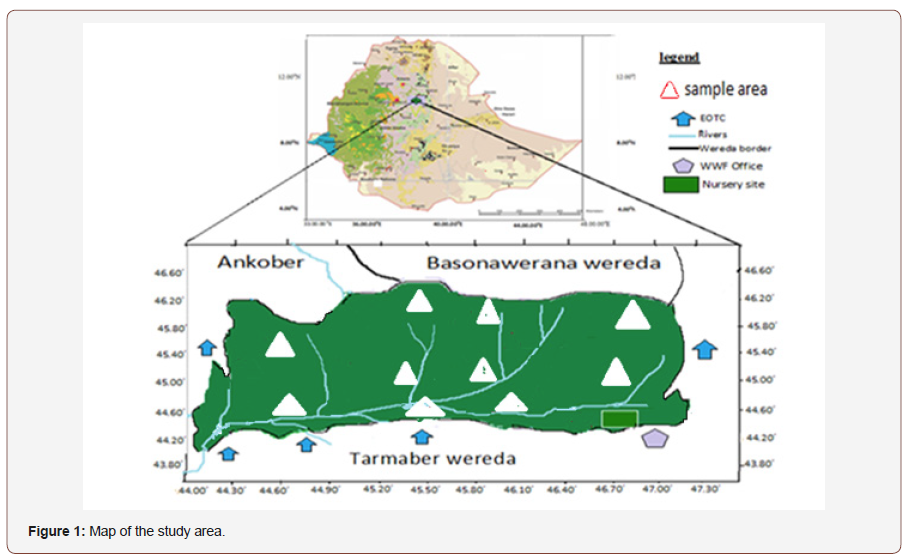
Sample collection
Soil samples were collected in a sterile polyethylene containerat the depth of 15 cm pH 4.8 temperatures 25° C which wasmeasured at the site of sampling area by using centimeter, pHmeter and thermometer respectively. The sampling technique wereselected randomly from the Northern, Southern, Eastern, Westernand Central part of the forest from ten sample area at 2 km intervalsand brought to the laboratory for further processing.
Isolation of fungi from soil sample
Fungal colonies were isolated by diluting 10 gm of soil samplesin 100 ml of sterile distilled water by serial dilution method. Potatodextrose agar (PDA) (Potato 200 gm, Dextrose 20 gm, Agar 20gm, Distilled water 1000 ml) media was prepared, autoclaved andpoured in sterile Petri plates and 0.1 ml of soil samples were dilutedup to 10-5 dilutions and spread on respective solidified PDA plates.The inoculated plates were incubated at ambient temperature for120 hours (5 days).
Fungal isolates were differentiated on the basis of their physicalcharacteristics such as hyphae and mycelium, which was obtainedafter incubation. Successive purification was carried out on sterilePDA plates by point inoculation and incubated by following theabove method until pure isolate was obtained. Pure isolates werestored at 4° C using slants for further work [9].
Screening of fungal isolates for amylase production
To select the best amylase producing strains 108 fungal isolateswere grown in starch agar media comprising the following, in gmL 1; yeast extract 1.5, peptone 0.5, sodium chloride 1.5, starch 10, agar15, and make the media of the pH similar with pH of soil sample.The isolates were inoculated on sterile solidified starch agar platesa blank without inoculation was also maintained for comparison.Then the plates were incubated at room temperature for 120 hoursafter that all the plates along with blank was flooded with iodineand observed for zone of hydrolysis [10].
Inoculums preparation
Inoculums were prepared by adding 10 ml of sterilizeddistilled water into a sporulated 5 days old PDA slant culture. Aninoculums needle was used to displace the spore clusters understerilized conditions and then it was shaken thoroughly to preparehomogenized spore suspension [11].
Production of crude amylase using solid statefermentation
Solid state fermentation (SSF) medium was used for enzymeproduction from selected isolates using wheat bran as a sole carbonsource supplemented with the following nutrients g/100 ml (%):glucose, 0.05; NH4SO4, 0.02; trisodium citrate, 0.027; MgSO4, 0.008;KH2PO4, 0.19 and 10g of wheat bran was moistened with abovenutrient solution to a level of (v/w) 65% in 250 ml size Erlenmeyerflasks was used for cultivation of the fungal isolates. The moisturelevel of the wheat bran was removed after overnight oven drying at105° C. To extract the released enzyme the moldy bran was soakedin 10 mM acetate buffer pH 4.8 in (w/v) 1:10 ratio [12], and shackedfor 1hr at 120 rpm on the shaker, then the crude enzyme from SSFmedium was harvested by centrifugation repeatedly at 5,000 rpmfor 10 min [13].
Amylase assay
Enzyme assay was carried out based on the dinitro salicylicacid method [14]. As a substrate one gram of soluble starch wasdissolved in 100 ml 50 mM acetate buffer pH 4.8 and gelatinized on aheater. Dinitro salicylic acid (DNSA) solution standard compositionwas prepared: g/l phenol 2; sodium sulfite 0.5; sodium potassiumtartarate 200; Sodium Hydroxide (NaOH) 10; and Dinitro salicylicacid 10. To a 0.9 ml of the substrate 0.1 ml of enzyme source wasadded and incubated for 10 min at 50° C in water bath. At the end ofthe incubation period the reaction was stopped by adding 2 ml DNSsolution. Enzyme source was added into a control after the reactionwas stopped by the DNS solution. Then the reaction mixture wasboiled for exactly 5 min in boiling water. Finally, the test tubes werecooled in running water for about 5min and the optical density(OD) of the resulting colored solution was measured at 540 nmagainst a blank.
Enzyme characterization
Identification of amylase type by sugar analysis on thinlayer chromatography (TLC):To identify the type of amylase fromthe fungal isolates based on the starch hydrolysates TLC system[15-16] followed. First 0.9 ml 2% soluble starch mixed with 0.3mlcrude enzyme from fungal sources was incubated for 30min at 50°C in the water bath. Then the hydrolysate was spotted on TLC platealong with standard known sugar (glucose, maltose and dextrose)solution. A one dimensional ascend was done by using a solventsystem (v/v) of butanol: ethanol: water (5:3:2). After a total of fiveascends air-dry TLC plates were sprayed with 50% (v/v) Methanol-H2SO4 heated for 10 min at about 100° C. The sugar spots appearedwas identified by comparing with the standard known sugar.
Effect of pH on activity and stability of amylase: Todetermine the pH activity of the fungal amylases the crude enzymewas assayed using 1% soluble starch prepared in an acetate buffersystems pH 2.0 to 11.0. The activity of enzyme was determined byusing the standard assay conditions [14].
Effect of temperature on activity and stability of amylase: To determine the effect of temperature on the activity of fungalamylase, the crude enzyme was mixed with starch soluble in 50mM acetate buffer of pH 4.8 and incubated at temperatures of 20°C, 30° C, 40° C, 50° C, 60° C, 70° C, 80° C and 90° C for 10 min. Thenthe activity of amylase enzyme was determined by standard assaymethod Millern HP [13].
Effect of sodium ion concentration on amylase activity:Todetermine effect of metal ion on amylase activity the crude enzymewas mixed with different sodium ions concentration (0 M, 0.5 M,1 M, 1.5 M, 2 M and 2.5 M) together with soluble starch in 50mMacetate buffer pH 4.8 and the activity of the enzyme was determinedby standard assay method as mentioned above [17].
Effect of culture condition on enzyme production:Differentincubation periods, moisture levels and carbon and nitrogenssources were used to determine the amount of amylase produced.Selection of appropriate carbon and nitrogen sources or othernutrients is one of the most significant stages in the development ofan efficient and cost-effective process [18].
Effect of incubation periods on amylase production:Todetermine the time for maximum enzyme production the isolatewas allowed to grow in solid state fermentation and the study wascarried out at different incubation periods such as 24, 48, 72, 96,120 and 144 hrs, after which amylase production was determinedby standard assay method [19].
Effect of moisture levels on amylase production:Theoptimum moisture level for enzyme production was determinedby growing the isolates at 28° C in the SSF media at a moisturelevel (v/w) of 45%, 55%, 65%, 75% and 85%). The enzyme washarvested, and its activity was determined by standard assaymethod [20].
Effect of carbon sources on amylase production:The effectof carbon source on growth and enzyme production by the isolatewas determined by growing the test organism in SSF medium(by the method mentioned earlier) in which 0.5% of either ofthe following carbon sources: glucose, sucrose, lactose, fructose,starch and wheat flour were used as a sole carbon source. After harvesting the enzyme standard assay was carried on to determineeffect of carbon sources on enzyme production. One unit of enzymeactivity was defined as the number of micromoles of reducing sugarproduced per minute in the reaction condition.
Effect of nitrogen sources on amylase production:Todetermine the appropriate nitrogen source for amylase productionby the isolate, the test organism was cultivated in SSF medium(by the method mentioned in section 3.6) in which 0.2% (w/v) ofeither of the following nitrogen sources: NH4Cl, NH4NO3, (NH4)2SO4,peptone, tryptone, and urea used as a sole nitrogen source. Thenthe crude enzyme was harvested and assayed to determine effect ofnitrogen sources on amylase production.
Results and Discussions
Isolation and screening of amylolytic fungi
Screening of fungal isolates for amylase production was doneby starch hydrolysis test and the result indicated that 25% (27)of them are positive for amylase production. Further screening ofthese isolates which had relatively wider clear zone around thecolony resulted in the selection of nine best isolates of amylaseproducing strains.
To select the best amylase producing fungi all the nine amylolyticisolates which had relatively wider clear zone were grown in liquidmedium and the level of amylase activity was determined from thecell free culture supernatant. A total of 3 isolates that producedappreciable amylase activities in liquid culture were selected onthe bases of the area of clearance and OD results of supernatant.Then, the best enzyme producer was selected for further studieson amylase production [21]. Due to maximum relative activity ofenzyme in temperature and pH (Tables 1 and 2) and the formationof wider clear zone as compared to the other, isolate 46 was selectedfor further study.
Table 1: Effect of temperature on the activity of Amylase for threeselected fungal isolates..
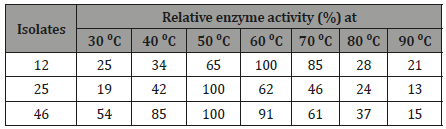
Table 1: Effect of pH on the activity of Amylase for three selected fungalisolates..

Morphological characterization of the isolate
The fungal isolate used in this study had a macroscopic featureon petri dish containing PDA media showed rapid growth; darkgreen color with yellowish border and the colony have granularpowdery appearance. The back side of the colony was paleyellow in color and microscopic feature showed septate hyphaeand conidiophore was attached to the septate. Based on thesemorphological features this isolate was tentatively grouped underthe genus Penicillium [22].
Identification of amylase type by TLC
The amylase that was produced from the selected fungal isolate(WWFI 46) allows producing starch hydrolysates and runningon TLC plate along with glucose, dextrose and maltose standardsolutions. The fungal amylase mainly developed spot that wasparallel with the standard glucose showed that the test enzymewas glucoamylase. This was direct evidence for the presence ofglucoamylase in the fungal extract that resulted in the hydrolysis ofstarch to glucose. A similar analysis was reported elsewhere [23].
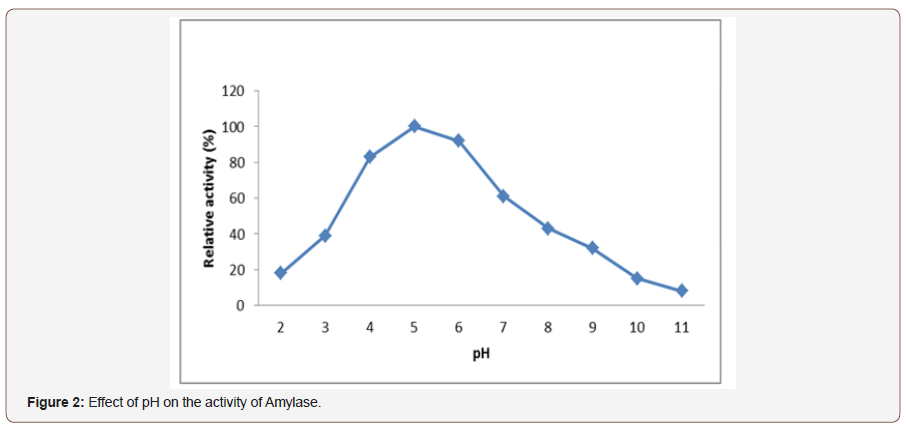
Effect of pH on activity of amylase:To determine the effectof pH on the activity of the glucoamylase enzyme incubated in apH range 2.0 to 11.0 (at one interval). In this study as the pH valueincrease amylase activity also increases and its highest activity wasobserved at pH 5.0. The maximum amylase activity was found atpH 5 (Figure 2). Further increase in the pH resulted decrease in theactivity of amylase. Different organisms have different pH optimaand decrease or increase in pH on either side of the optimum valueresults in poor microbial growth, the decreased activity in thelater phase of growth was probably due to catabolic repression byglucose released from starch hydrolysis. Similarly, [24] reportedthat fungal amylase could be denatured at high pH value.
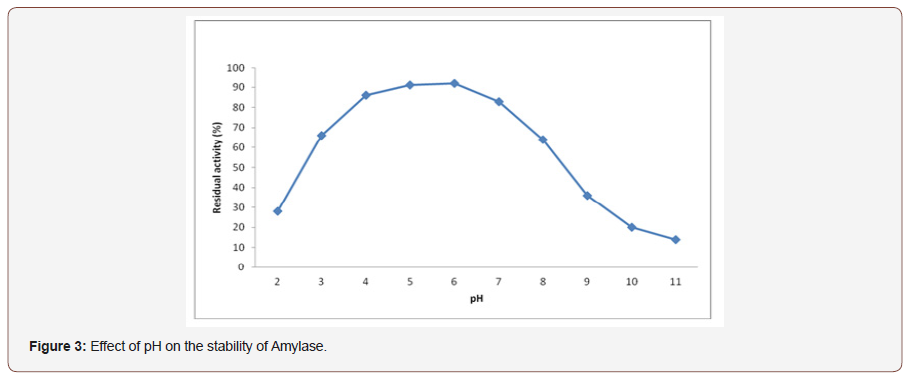
Effect of pH on stability of amylase:The production ofamylase in the fermentation medium affected by different pH valueand have very essential role in the synthesis, secretion and stabilityof amylase [25]. After 1hr of incubation pH stability of fungalamylase was evaluated by assaying the enzyme and showed broadstability to pH range of 4.0 to 7.0 in which the enzyme retained aresidual activity of at least 83% (Figure 3). This may be taken asan advantage for enzymatic liquefaction of starch industriallyat moderately acidic condition (pH around 5). As a result, it canavoid problems that would pose undesired byproduct formation,high cost of product recovery in the downstream processing, andreduced amount of yield. It was also reported [26] that as pHvalue increased beyond 7.0, the enzyme stability showed a gradualdecline due to the accumulation of hydroxyl ions.
Effect of temperature on activity of amylase:The resultshowed that as temperature increases amylase activity alsoincrease at a certain temperature. The highest relative activityof amylase was observed in a range of 40-600C and reached itsmaximum activity at 500C. There was a decrease in enzyme activitywhen the incubating temperature was further increased beyondthe maximum and the least activity also observed at 900C (Figure4). Temperature above 50°C result in moisture loss of the substrate,affect metabolic activities, reduction in growth and enzymeproduction of microorganism. As reported [27] that due to hightemperature, the growth of the fungal isolate was greatly inhibitedand hence, enzyme formation was also prohibited [28,29].
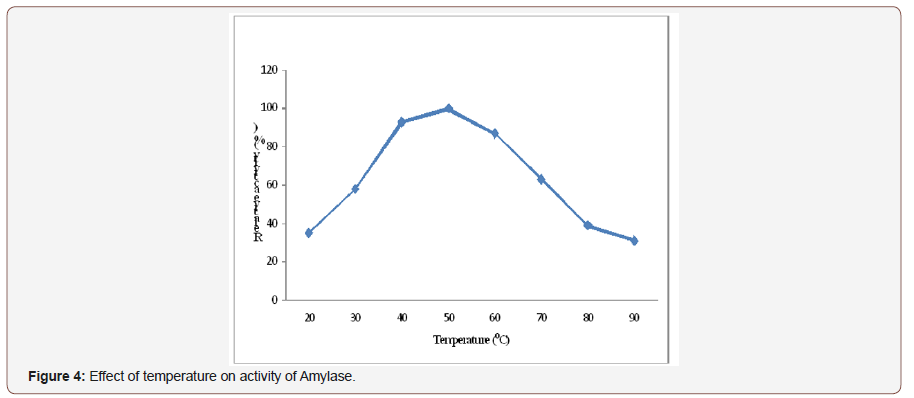
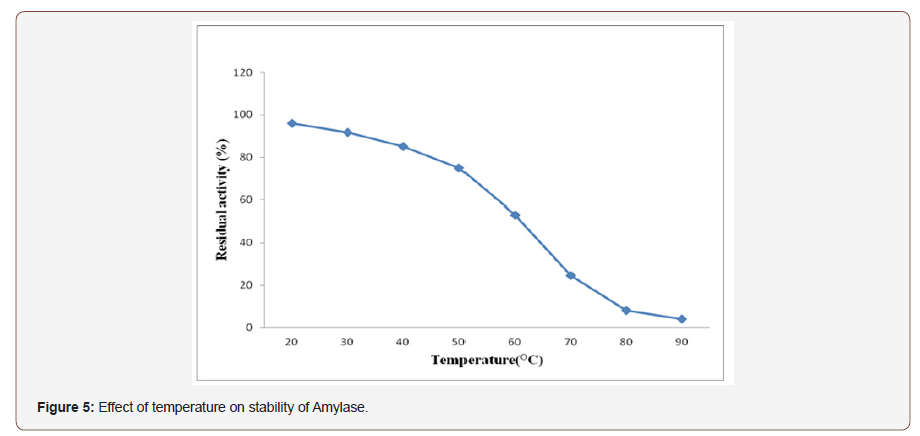
Effect of temperature on stability of amylase:Temperaturestability of amylase was determined by incubating the enzyme ata range of (20-90° C) for 30min and followed the standard assaymethod of amylase. The result shows that amylase retained a residualactivity of 96%, 91.8% and 85% related to incubation temperatureof 20, 30 and 40 °C for 30 min respectively. It maintained more than50% of its residual activity after it was incubated at temperaturesbelow 60° C for 30min. It also maintained about 75% of its originalresidual activity at its maximum temperature of activity (50° C)after 30 min of incubation (Figure 5). The residual activity ofthis enzyme decreased with increase in incubation temperature.Furthermore, it was reported [30] that at high temperature, thestability of the enzyme was greatly affected and hence, enzymeproduction also reduced.
Effect of culture condition on amylase production
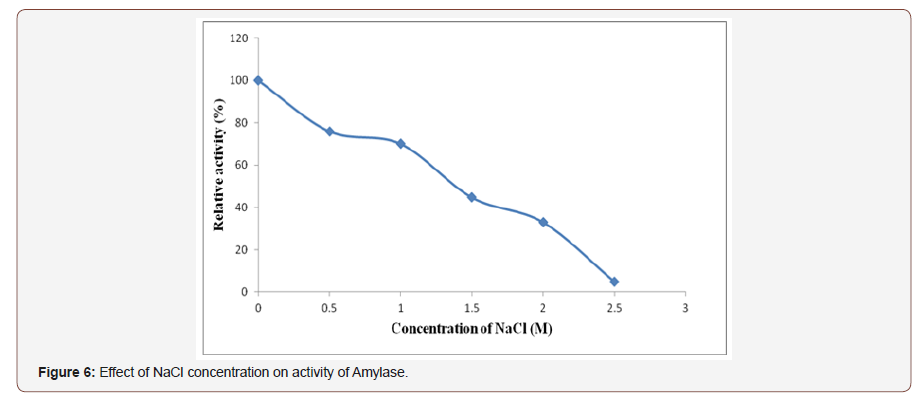
Effect of sodium ion (Na+) concentration on amylaseactivity:The enzyme activity in the presence of different sodiummetal ions concentration was analyzed and it was found that thehighest (100%) enzyme activities occurred in the absence of NaCland subsequently decrease in their activity as the sodium ionconcentration increases (Figure 6). Similarly, the activity of amylasewas lost when NaCl concentration increased [31]. This might bethe distraction of ion linkages due to the interaction of enzymewith sodium ion at the surface of their natural structure there byresulted in inhibiting and salting of the enzyme active site [32].
Effect of incubation period on amylase production:Fungalamylase from selected isolate was produced by using solid-satefermentation at different time intervals ranging from 24, 48, 72,96, 120 and 144 hrs incubation. As the incubation time increaseamylase activity also increase and maximum amylase productionwas obtained at 72 hours of incubation. Beyond this period relativeactivity of enzyme was decreased (Figure 7). Time course studyfor the production of amylase governed by characteristics of theculture and also based on growth rate and enzyme production [33].
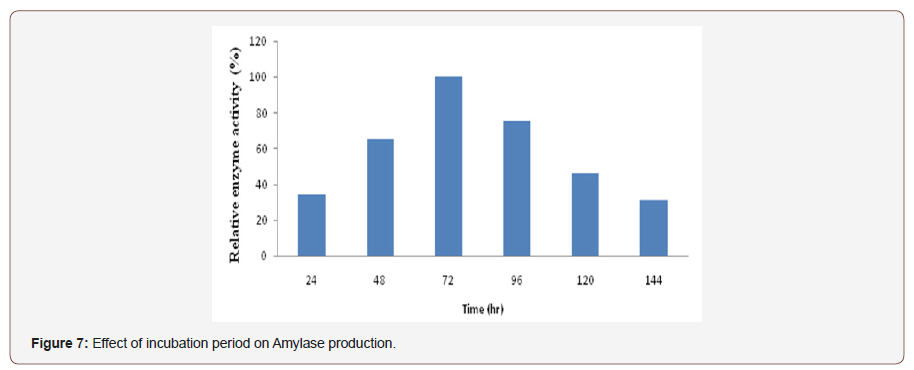
Enzyme secretion usually occurs maximally during the lateexponential and early stationary phases. A prolonged incubationtime beyond this period did not increase the enzyme yield ratherdecrease their secretion and reaches stationary phase andcould have started producing secondary metabolites and loweryield of enzyme. The reason for this might have been due to thedenaturation of the enzyme caused by the interaction with othercomponents in the medium, deficiency of nutrients, accumulationof toxic substances and proteolysis of amylase as reported by manyworkers [34].
Effect of moisture level on production of amylase:Enzymeproduction was affected by the moisture content of the substratethat was grown in SSF. To determine the maximum enzymeproduction the selected fungal isolate was grown on wheat brancontaining different moisture contents. The result indicates thatthe least amount of amylase was produced in wheat bran substrateat moisture content of 45% and the maximum amylase yield wasobserved at 65% of moisture content. Increase in moisture contentbeyond 65% leads to reduction in product yield (Figure 8). This isdue to as moisture level increased anaerobic condition could becreated, water molecules occupy the air space in the wheat bran,decreases porosity of solid state media, changes wheat bran particlestructure, reduces gas volume, decreases diffusion and clumping ofthe solid particles resulted subsequent reduction in enzyme yield[35]. Generally, the higher moisture content leads to high catabolicrepression because water activity increases rate of glucosediffusion. Low moisture content also reduces microbiologicalactivity, solubility and better utilization of nutrients [36].
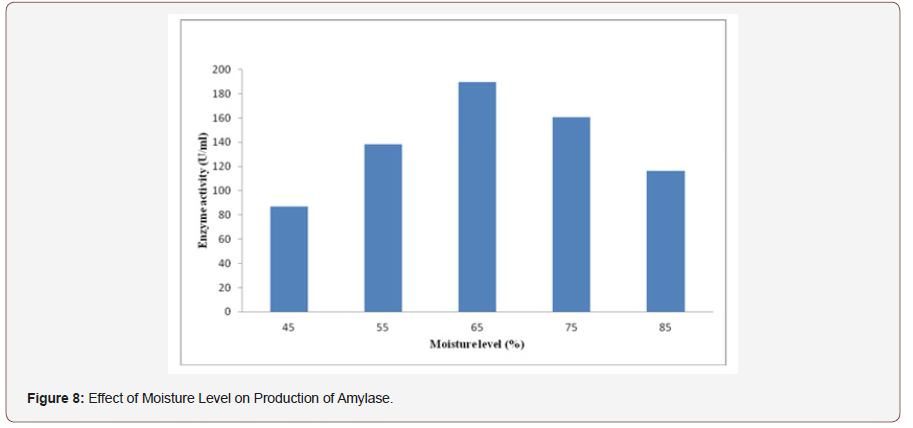
The production of enzyme is greatly dependent on the conditionof growth of the culture and composition of nutrient medium[37,38]. In SSF, most of the microbial growth and product formationtakes place at or near the surface of the solid substrate. Thus, itis very crucial to provide optimized water level that controls thewater activity of the fermenting substrate for achieving maximumproduct [39].
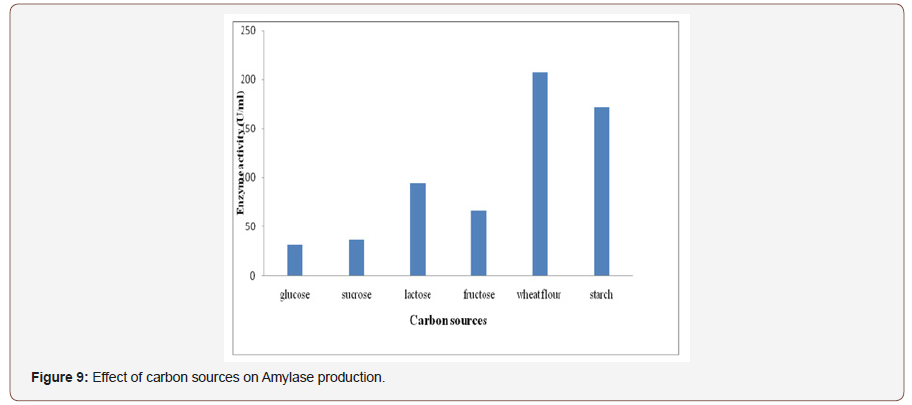
Effect of carbon sources on amylase production:Thenature and amount of carbon source in culture media is importantenergetic source for the production of extracellular amylase fromfungal isolate (Figure 9). Therefore, production of amylase wastested by growing in SSF containing different carbon source as asubstrate [40]. To determine amylase production supplementationof various carbon sources in growth media requires in the form ofmonosaccharide’s (Glucose and Fructose), disaccharides (Lactoseand Sucrose) and polysaccharides (Starch and Wheat flour). Wheatflour was the best carbon source for amylase production whereasglucose results the least enzyme product and the other testedcarbon sources gave comparatively less enzyme production, whichis supplemented to the fermentation medium [41]. Therefore,soluble starch and wheat flour was optimized as a carbon sourcefor further experimental work. Many other workers also reportedwheat flour as the best carbon source for amylase production [42].Different carbon sources have different influence on the productionof extracellular enzymes [43].
Effect of nitrogen sources on amylase production:Nitrogenis the secondary energy sources and plays an important role in thegrowth of the organism and enzyme production. The nature of thecompound and the concentration that we used might stimulate ordown reduce the production of enzymes. In this study the effect ofsupplementary organic nitrogen sources (Peptone, Tryptone, Urea)and inorganic nitrogen sources (NH4)2SO4, NH4NO3, NH4Cl) on theproduction of amylase by fungal strain using SSF was determined(Figure 10).
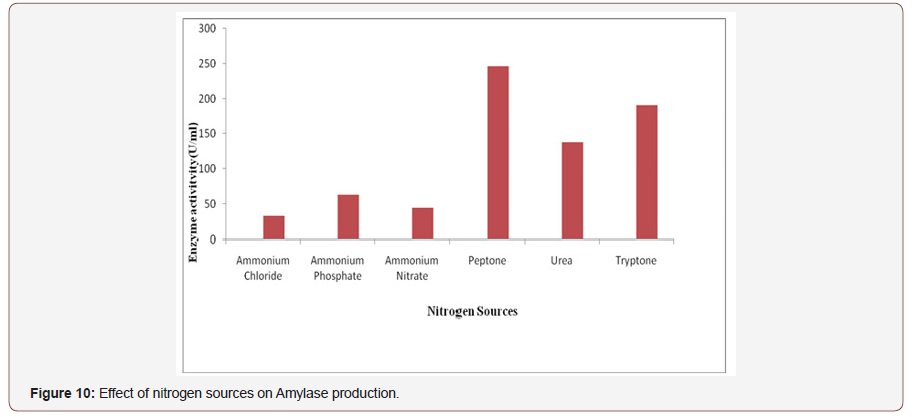
Among all the selected inorganic nitrogen sources maximumamylase production was obtained, when (NH4)2SO4 was added inthe medium [44]. In case of organic nitrogen sources peptone wasfound the highest for amylase production, probably due to its highcontent in minerals, vitamins, coenzymes and nitrogen components[45]. Many workers reported that peptone as an organic nitrogen source produces maximum amylase. NH4NO3, NH4Cl and (NH4)4SO4showed inhibitory effect on enzyme production. It might be due tothe pH changes associated with their use in the medium [46].
Conclusion
The overall finding of the present study shows that it is possibleto isolate amylase producing fungi from forest area like WofWasha forest. Additionally, the isolated fungi strains were foundto belong to penicillium genus, as these groups of fungi known toproduce amylase. Maximum enzyme was produced from selectedisolates using wheat bran as a sole carbon source which is easilyavailable everywhere from agricultural waste within inexpensivecost. Enzyme produced from the isolated fungal strain had beenstabilized with changes of temperature and pH on the activityand stability of amylase have taken as an advantage for enzymaticliquefaction of starch industrially at moderately acidic condition ofpH around 5. The strain also showed maximum enzyme productionin lower temperature ranges. This perhaps helps the strain not tobe affected by evaporation and produce the enzyme without anyneed of incubator and humidifier. The strain grew in a short time ofincubation. This is important to get the enzyme in very short timeand thus reducing the cost of production and risk of contamination.
Amylolytic property of the enzyme helps to convert starchinto smaller unit and value-added product for human and animalconsumption. The enzyme is highly active at optimum temperatureof 50° C and pH 5. It is also active in broad pH and temperatureranges. The enzyme is highly efficient for starch hydrolysis. Allthese properties infer that its suitable application in starch andstarch product industry. The enzyme has a great potential for starchindustry because of its amylolytic potential and environmentallyfriendly approach.
Acknowledgment
We would like to acknowledge the Director of Post GraduateStudies of Haramaya University and Science Faculty of Addis AbabaUniversity for financial support and the local communities for theirvarious supports and valuable information in this study. Thanks tothe Central Laboratory staff for their support during data analyses.
Conflict of Interest
No conflict of interest.
References
- Kirk O, Borchert TV, Fuglsan CC (2002) Industrial enzyme applications. Curr Opin Biotechnol 13(4): 345-351.
- Negi S, Banerjee R (2010) Optimization of culture parameters to enhance production of amylase and protease from Aspergillus awamori in a single fermentation. African Journal of Biochemistry Research 4(3): 73-80.
- Chengyi WH, Ming MM, Jiang R (1999) Studies on the properties of alpha amylase produced by Bacillus pumilus 289 (PBX96). Acta Microbial Sin 32(6): 400-404.
- Merheb H, Cabral E, Gomes F, Da Silva R (2007) Thermophilic amylase production from the soil. Niger J Sc Technol 11(1): 30-38.
- Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem 280 (Pt 2): 309-316.
- Kunamneni A, Permaul K, Singh S (2005) Amylase production in solid state fermentation by the thermophilic fungus Thermomyces lanuginosus. J Biosci Bioeng 100(2): 168-171.
- Raimbault P (1998) General and microbiological aspects of solid substrate fermentation. Electron J Biotechnol 3: 1-15.
- Mamo G, Gessesse A (1999) Effect of cultivation conditions on the growth and α-amylase production by a thermophilic Bacillus sp. Lett Appl Microbiol 29: 61-65.
- Gillman CJ (1998) A manual of Soil Fungi Biotech Books, India, Pp. 235.
- Kumar, Duhan JS (2011) Production and characterization of amylase enzyme isolated from Aspergillus niger MTCC-104 employing solid state fermentation. Int J Pharma and Bio Sciences 2(3): 250-258.
- Lingappa K, Vivekbabu CS (2005) Production of lovastatin by solid state fermentation of carob (Ceratoniasiliquq) pods using Aspergillus terreus KLVB28. Ind J Microbiol 45(4): 283-286.
- Omemu AM, Akpan I, Bankote MO, Tenioda OD (2005) Hydrolysis of raw tuber starches by alpha amylase of A. niger isolated from the soil. Afr J Biotechnoi l4(1): 19-25.
- Soni S, Kaur K, Gupta JK (2003) A solid state fermentation based bacterial alpha amylase and fungal glucoamylase synthesis and its suitability for hydrolysis of wheat starch. Proc Biochem 39: 185-192.
- Millern HP (2010) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 52: 324-531.
- Kimura A, Horikoshi M (1998) How do histone acetyltransferases select lysine residues in core histones. FEBS Lett 431(2): 131-133.
- Mamo G, Gessesse A (1999) A highly thermostable amylase from a newly isolated thermophilic Bacillus sp. WN11. J Appl Microbiol 86: 557-760.
- Tunga R, Tunga BS (2003) Extracellular amylase production by Aspergillus oryzae under solid state fermentation. Japan International centre for Biochemistry pp. 1-12.
- Konsoula M, Kyriakides L (2007) Co-production of α-amylase and β-galactosidase by Bacillus subtilisin complex organic substrates. Bioresource Technol 98(1): 150-157.
- Bertrand TF, Fredric T, Robert N (2004) Production and partial characterization of a thermostable amylase from Ascomycetes yeast strain isolated from starchy soil. Mc Graw-Hill Inc, USA, pp. 53-55.
- Ellaiah P, Adinarayana K, Bhavani Y, Padmaja P, Srinivasulu B (2002) Optimization of process parameters for glucoamylase production under solid state fermentation by newly isolated Aspergillus species,” Process Biochemistry 38(4): 615-620.
- Abe J, Bergman FW, Obeta K, Hizukuri S (1998) Production of the raw starch degrading amylase of Aspergillus sp. K- 27. Appl Microbiol Biotechnol 27(5-6): 447-450.
- Tiwari KL, Jadhav SK, Fatima A (2007) Culture condition for the production of thermostable amylase by Penicillium rugulosum. Global J Biotechnology and Biochemistry 2(1): 21-24.
- Goto CE, Barbosa EP, Kistner LC, Moreira FG, Lenartovicz V, et al. (1998) Production of amylase by Aspergillus fumigatus utilizing alpha-methyl-D- glycoside, a synthetic analogue of maltose, as substrate. FEMS FEMS Microbiol Lett 167(2): 139-43.
- Ramesh MV, Lonsane BK (1991) Regulation of alpha-amylase production in bacillus licheniformis M 27 by enzyme end-products in submerged fermentation and its overcoming in solid state fermentation system. Biotechnol Letters 13(5): 355-360.
- Sindhu GS, Sharma P, Chakrabarti T, Gupta JK (1997) Strain improvement for the production of a thermostable α-amylase. Enzy Microbiol Technol 21(7): 525-530.
- Dubnovitsky AP, Kapetaniou EG, (2005) AC Papageorgiou Enzyme adaptation to alkaline pH: Atomic resolution (1.08Å) structure of phosphoserine aminotransferase from Bacillusalcalophilus. Protein Sci 14(1): 97-110.
- Krishna C, Chandrasekaran M (1996) Banana waste as substrate for amylase production by Bacillus subtilis (CBTK 106) under solid-state fermentation. Appl Microbiol Biotechnol 46(2): 106-111.
- Pandey A, Nigam P, Soccol CS, Soccol VT, Singh D, et al. (2000) Advances in microbial amylases. Biotechnol Appl Biochem 31(pt 2): 135-152.
- Radley JA (1976) Starch Production Technology. Applied Science Publishers Ltd, UK, pp. 587.
- Balkan B, Ertan F (2007) Production of a-Amylase from Penicillium chrysogenum under Solid-State Fermentation by Using Some Agricultural By-Products. Food Technol Biotechnol 45(4): 439-442.
- Reyed MR (2007) Biosynthesis and properties of extracellular amylase by encapsulation Bifidobatrium bifidum in batch culture. Aust J Basic Appl Sci 1(1): 7-14.
- Zambare N (2010) Solid state fermentation of Aspergillus oryzae for Glucoamylase Production on Agro residues. Int J Life Sci 4: 36-45.
- Nusrat A, Rahman SR (2007) Comparative studies on the production of extracellular α-amylase by three mesophilic Bacillus isolates. Bangladesh J Microbiol 24(2): 129-132.
- Shafique S, Bajwa R, Shafique S (2009) Screening of Aspergillus niger and a. flavus strains for extra cellular alpha-amylase activity. Pak J Bot 41(2): 897-905.
- Lonsane BK, Ghildyal NP, Budiatman S, Ramakrishna SV (1985) Engineering aspects of solid-state fermentation. Enzyme Microb Technol 7(6): 258-265.
- Zadrazil F, Brunnet H (1981) Investigation on physical parameters important for the SSF of straw by white rot fungi. Eur J Appl Microbiol Biotechnol 11: 183-188.
- Cherry HM, Hossain MT, Anwar MN (2004) Extracellular glucoamylase from the isolate Aspergillus fumigatu. Pakit J Biol Sci 7(11): 1988-1992.
- Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial α-amylases: a biotechnological perspective. Process Biochemistry 38(11): 1599-1616.
- Haq I, Ashraf H, Qadeer MA, Iqbal J (2003) Production of alpha amylase by Bacillus licheniformis using an economical medium. Bioresour Technol 87(1): 57-61.
- Chimata MK, Sasidhar P, Challa S (2010) Production of extracellular amylase from agricultural residues by a newly isolated Aspergillus species in solid state fermentation. Afri J Biotechnol 9(32): 5162-5169.
- Narang S, Satyanarayana T (2001) Thermostable alpha amylase production by an extreme thermophile Bacillus thermooleovorans. Lett Appl Microbiol 32(1): 31-35.
- Aiyer PVD (2004) Effect of C: N ratio on alpha amylase production by Bacillus licheniformis SPT 27. African Journal of Biotechnology 3(10): 519-522.
- Heseltine C, Rolfsmeier M, Blum P (1996) The glucose effect and regulation of amylase synthesis in the hyperthermophilic archeon Sulfolobus solfataricus. J Bacteriol 178(4): 945-950.
- Hashemi M, Shojaosadati SA, Razavi SH, Mousavi SM, Khajeh K, et al. (2012) The efficiency of temperature shift strategy to improve the production of α-Amylase by Bacillus sp in a solid-state fermentation system. Food and Bioprocess Technol 5(3): 1093-1099.
- Pederson H, Neilson J (2000) The influence of nitrogen sources on alpha amylases productivity of Aspergillus oryzae in continuous cultures. Appl Microbiol Biotechnol 53(3): 278-281.
- Ashokkumar B, Kayalvizhi N, Gunasekaran P (2001) Optimization of media for fructofuranosidase production by Aspergillus niger in submerged and solid-state fermentation. Process Biochemistry 37(4): 331-338.
-
Negussie F Bussa, Mesfin Moges, Manikandan Muthuswamy, Melese Abdisa. Isolation and Characterization of Amylase Enzyme fromSelected Fungal Strains of Wof Washa Forest of North Shoa, Ethiopia. Sci J Biol & Life Sci. 1(2): 2019. SJBLS.MS.ID.000506.
Cyberattack, Violation, Privacy, Feelings, Impressions, Emotions, Trigger attitudes, Behaviors, Virtual space, Transverse zone, Cyberspace, Avatarization, Virtual intelligence, Cyberbullying, Epileptic
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






