 Research Article
Research Article
Immunomodulatory Effects of Bio-Clean II on Total and Differential Leucocyte Counts in Rats Exposed to Purified Bacterial Lipopolysaccharide
Seyi Samson Enitan1*, Isaiah Nnanna Ibeh2, Ayodeji Olusola Olayanju3, Emmanuel Olusegun Ileoma1, Abiodun Durosinmi4, Aderanti Adedotun Afeez5, Nkem Judith Adeyombo6, Ayomide Ruth Olabanji1
1Department of Medical Laboratory Science, Babcock University, Ilishan-Remo, Nigeria
2Department of Medical Laboratory Science, University of Benin, Nigeria
3Department of Medical Laboratory Science, Afe Babalola University, Ado-Ekiti, Nigeria
4Department of Haematology, State Hospital, Ijebu-Ode, Nigeria
5Department of Medical Laboratory Science, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
6Department of Haematology, Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria
Seyi Samson Enitan, Department of Medical Laboratory Science, Babcock University, Ilishan-Remo, Nigeria.
Received Date:May 20, 2022; Published Date: July 06, 2022
Abstract
Bio-Clean II has been previously shown to possess anti-inflammatory properties by regulating the serum level of inflammatory cytokines, C-reactive protein, Corticosterone, antiphospholipid antibodies, as well as T-Helper 4 and Cytotoxic T-Lymphocytes in rats exposed to purified bacterial lipopolysaccharide (LPS). In this study, we investigated the effects of Bio-Clean II on total and differential leucocyte counts in rats exposed to bacterial LPS. Thirsty six (36) male Wistar rats (150±50g) were divided into six (6) groups (n=6). Group 1, 2, 3, 5 and 6 were induced with a single dose of 5mg/Kg of purified Lipopolysaccharide from (E. coli O127:B8) intraperitoneally, except the Zero control (group 4) that was given feed and water only. The positive control (group 2) received 50mg/Kg/bid Diclofenac + 500mg/kg/bid of Ciprofloxacin, Negative control (group 3) received Sterile Phosphate Buffer Saline, while group 5 and 6 received Bio-Clean II for duration of 7 and 14 days, respectively. The rats were sacrificed by cervical dislocation afterwards, whole blood was collected and assayed for total white blood cell count (WBC), absolute lymphocyte count (LYM#), absolute neutrophil count (NEUT#), as well as absolute counts of the summation of monocytes, eosinophils and basophils (MXD#) using Sysmsex Haematology Autoanalyzer. The results of the study shows that the WBC (5.68±0.91 X103/uL), LYM# (4.04±0.85X103/uL), and NEUT# (1.10±0.11X103/uL) of the 7days (not 14 days) Bio-Clean II treated rat was found to be significantly lower when compared to the inflammation control group: 12.52±1.04 X103/uL (p=0.018), 7.66±0.75X103/uL (p=0.004) and 4.40±0.81X103/uL (p= 0.027), respectively. The outcome of this study shows that Bio-Clean II reversed the leukocytosis, lymphocytosis and neutrophilia associated with LPS-induced inflammation. This underscores the anti-inflammatory potential of Bio-Clean II in the treatment of bacterial inflammatory diseases. While data generated from this study may be extrapolated to humans, further studies are required to understand the molecular mechanism behind these effects.
Keywords: Bio-Clean II; Herbal remedy; Leucocyte counts; Lipopolysaccharides; Rats
Introduction
Bacterial inflammatory diseases have had an enormous impact on human society and despite the discovery of antibiotics, have continued to be a major threat to public health [1]. From the moment of birth, humans are constantly exposed to a wide variety of assaults, including those of bacteria origin [2]. Exposure to bacterial lipopolysaccharide (LPS) has been associated with inflammation of body tissues characterized by swelling, redness, pain, and loss of function if the assaulting agent persists. One of the consequences of bacterial infection is inflammation [3]. Inflammation may be caused by a variety of bacterial components. Lipopolysaccharides (LPS) in particular are potent inflammatory triggers. They are glycolipids and the primary component of the outer membrane of Gram-negative bacteria [4]. They contribute to the integrity of the outer membrane, and protect the cell against the action of bile salts and lipophilic antibiotics [5]. LPS is a prime target for recognition by the innate immune system and is responsible for the majority of inflammatory effects of infections from Gram-negative bacteria [6]. As an endotoxin, it is a potent immune activator which can lead to fatal septic shock syndrome if the inflammatory response is amplified and uncontrolled [4]. LPS is recognized by the innate immune system because it has a typical pathogen-associated molecular pattern that is recognized by tolllike receptor-4 (TLR4) on many cells including monocytes and macrophages [7]. Recognition of LPS is a complex process involving molecules that bind LPS and pass it on to cell membrane associated receptors on leukocytes, endothelial and other cells, which initiate pro-inflammatory cascade. The removal of the initial stimulus, a decrease in pro-inflammatory mediators (primarily cytokines and chemokines), the elimination of damaged and inflammatory cells, and the promotion of repair are all factors that contribute to the natural resolution of inflammation [3,8,9]. The inflammatory response involves a highly coordinated network of many cell types. Activated macrophages, monocytes, and other cells mediate local responses to tissue damage and infection. At sites of tissue injury, damaged epithelial and endothelial cells release factors that trigger the inflammatory cascade, along with chemokines and growth factors, which attract neutrophils and monocytes [10]. The first cells attracted to a site of injury are neutrophils, followed by monocytes, lymphocytes (natural killer cells, T cells, and B cells), and mast cells [11]. Monocytes can differentiate into macrophages and dendritic cells [12], and are recruited via chemotaxis into damaged tissues. Inflammation-mediated immune cell alterations are associated with many diseases, including asthma, cancer, chronic inflammatory diseases, atherosclerosis, diabetes, and autoimmune and degenerative diseases. Neutrophils, which target microorganisms in the body, can also damage host cells and tissues [10]. Neutrophils are key mediators of the inflammatory response, and program antigen presenting cells to activate T cells and release localized factors to attract monocytes and dendritic cells [13]. Traditional medicines, including the use of herbs, were the primary treatment for nearly all diseases in Africa even before the arrival of conventional medicine. Despite the widespread use of modern medicine in Nigeria today, herbal medicine is still widely used, and many people continue to depend on it for their health care [14]. A number of indigenous herbal remedies are in use today. Bio-Clean II herbal remedy for instance has been shown in a few studies to improve immunity and combat viral infections including HIV. It has been reported to work well on HIV patients and to show signs of complete tissue restoration. Specifically, it has been shown to increase CD4 Cell Count and body weight while lowering viral loads in a group of HIV-positive women [15,16]. Bio-Clean II has also been previously shown to possess anti-inflammatory properties by regulating the serum level of inflammatory cytokines [17], C-reactive protein, Corticosterone, antiphospholipid antibodies [18], as well as T-Helper 4 and Cytotoxic T-Lymphocytes [19] in rats exposed to purified bacterial lipopolysaccharide (LPS). Many of the preventive and therapeutic effects of Bio-Clean II are directly or indirectly attributable to its immunomodulatory properties [20] due to the presence of some important phytochemicals including resin, alkaloids, saponins, anthraquinones, tannin, and Cardiac Glycoside as reported in our previous studies [21]. To the best of our knowledge, this study is the first to investigate the effect of Bio-Clean-II on total and differential white blood cell counts in experimental LPS-induced inflammation. Scarcity of data in this regard, necessitates this study.
Materials and Methods
Study Design: Animal models are used in this analytic experimental investigation.
Duration of Study: The research work lasted for a period of 3 months (April-July, 2021).
Study Area: The research was conducted at Babcock University’s Experimental Animal House in Ilishan-Remo, Ogun State.
Source of Bio-Clean II: Bio-Clean II was procured from the manufacturer/maker on demand.
Source of Purified Lipopolysaccharide: As lyophilized powder, purified Lipopolysaccharide® (E. coli 0127:B8, Sigma- Aldrich, St. Louis, MO, USA) was purchased in vials. It was reconstituted in sterile Phosphate Buffer Saline (PBS) and given as a single dose (5 mg/kg) intraperitoneally.
Standard Drugs: Diclofenac (50mg) and Ciprofloxacin (500mg) were purchased from the Pharmacy Unit, Babcock University Teaching Hospital, Ilishan-Remo, Ogun State. Twenty (20) mL of Ciprofloxacin and Diclofenac suspension was prepared daily by dissolving one tablet each in 20 mL of sterile distilled water to obtain a concentration of 25mg/ml of Ciprofloxacin and 2.5mg/ ml of Diclofenac.
Table 1: Experimental pharmacological protocol.
Experimental Animals: A total of 36 male Wistar rats weighing 150g±50g (mean±SD) were purchased from the small animal house, University of Ibadan (Oyo State, Nigeria) and were clinically examined upon arrival for any signs of abnormality or disease. The animals were housed in the Experimental Animal House, Department of Animal Science, School of Agriculture and Industrial Technology, Babcock University, Ilishan-Remo (Ogun State, Nigeria) separately in well ventilated wire-bottom steel cages under hygienic conditions, with proper aeration at 25±2ºC, and a relative humidity of 45–50%. The rats were randomly assigned into 6 groups of six (6) rats each and were fed on standard rat diet (10g/100g body weight) twice daily and tap water ad libitum. Prior to commencement of the study, the rats were allowed to be stabilized in the Animal House with standard 12-hour light-dark cycle, for a period of 14 days. All studies on animal experimentation were conducted in accordance with the Current Animal Care Regulations and Standards approved by the Institute for Laboratory Animal Research [22]. The experimental animals were treated as shown in the experimental pharmacological protocol below (Table 1). Group 1 received a single dose of 5mg/Kg of purified LPS (Inflammation control). Group 2 received 5mg/Kg of purified LPS, 50mg/Kg/bid Diclofenac and 500mg/kg/bid of Ciprofloxacin (Positive Control). Group 3 received 5mg/Kg of purified LPS and sterile Phosphate Buffer Saline (Negative control). Group 4 were not exposed to purified LPS, rather they received water and feed only throughout the experimental period (Zero Control). Group 5 and 6 were treated with Bio-clean II for 7 and 14 days, respectively, post-exposure to purified LPS. Therapy with Bio-clean II was initiated by day 2 post- LPS challenge (Table 1).
The volume of Bio-clean II suspension given to each rat orally through an intragastric tube was determined and recorded prior to the start of the treatment period. After then, the rats were checked daily for any alterations. For the number of days stipulated in the experimental protocol, the study animals were administered a suitable volume of suspension (2ml/kg body weight) twice daily, every 12 hours (between the hours of 6.00-6.30 AM/PM). Before administration, the suspension was gently shaken and delivered via oral gavage. At days 8 and 15, the rats were sacrificed by cervical dislocation, as described by Ochei and Kolhatkar [23] and cardiac blood samples were collected for evaluation of total and differential white blood cell counts.
Determination of Total and Differential White Blood Cell Counts : Total and differential White blood cell counts were estimated using the Sysmsex® Automated Haematology Analyzer KX-21N, Sysmex Corporation (Kobe-Japan), as described by Samuel, et al. [24].
Principle: The equipment uses the principle of the laser flow cytometry. A single beam passes through a laser beam. The absorbance is measured and the scattered light is measured at multiple angles to determine the cells granularity diameter and inner complexity. These are the same cells morphology that can be determined manually from a slide.
Procedure
Briefly, the automated blood cell analyzer was switched on and the sample details and the identity number were entered. The sample were mixed gently and ensured that the tip of the sample probe was well placed into the tube so that it would touch the blood and the aspirate key was pressed. The tube was then removed when the sample probe comes out of the sample tube which indicates the end of aspiration. After the analysis of the sample, the result was printed out. The above procedure was repeated for other samples.
Data Analyses: Data was presented as means of 6 rats using bar charts. Data was analyzed using one-way analysis of variance (ANOVA) and Tukey-Kramer Multiple Comparisons Test using SPSS-20.0 (Statistical packages for social Scientists – version 20.0) statistical program. P values<0 was considered significant [25].
Results
This present study assessed the effects of Bio-Clean II on Total and Differential White Blood Cell Count in LPS-induced inflammation in animal model.
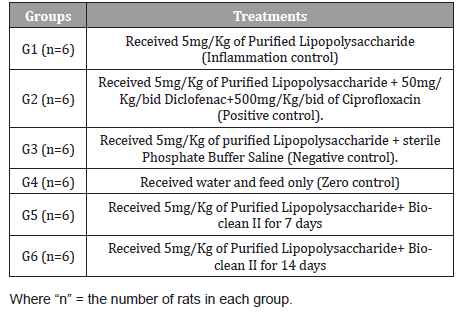
Effect on Total Leucocytes Count: The effect of Bio-Clean II on Total White Blood Cell counts in rats exposed to purified bacterial lipopolysaccharide is presented using a bar chart (Figure 1). The white blood cell (WBC) count of the 7days (5.68±0.91 X103/μL) Bio-Clean II treated rat was significantly lower (p=0.003) when compared to the inflammation control group (12.52±1.04 X103/μL); whereas, the 14days (10.80±1.16 X103/μL) Bio- clean II treated rat was found to be non-significantly lower (p=0.996). Also, the white blood cell (WBC) count of the 7days (5.68±0.91 X103/μL) Bio-Clean II treated rats was found to be non-significantly lower (p=0.879) when compared to the positive control group (7.42±0.31 X103/μL), whereas, the 14days (6:10.80±1.16 X103/μL) Bio-Clean II treated rats were found to be non-significantly higher (p=0.472). But on the other hand, 7days (5.68±0.91 X103/μL) Bio-Clean II treated rats were found to be non-significantly lower (p=0.762) when compared to the negative control group (10.18±2.01 X103/μL), whereas the 14days (10.80±1.16 X103/μL) Bio-Clean II treated rats were found to be non-significantly higher (p=1.000). Furthermore, the WBC count of the 7days (5.68±0.91 X103/μL) Bio-Clean II treated rats were found to be non-significantly lower (p=0.636) when compared to the Zero control group (7.96±0.36 X103/μL); whereas, the 14days (10.80±1.16 X103/μL) Bio-Clean II treated rats were found to be non-significantly higher (p=0.661). In addition, the WBC count level of 14days Bio-Clean II treated rats (10.80±1.16 X103/μL) was found to be non- significantly higher (p=0.130) when compared to 7days Bio-clean II treated rats (5.68±0.91 X103/μL). Meanwhile, the WBC count of the positive control (7.42±0.31 X103/ μL) was found to be non-significantly lower (p=0.985) than that of the negative control group (10.18±2.01 X103/μL) (Figure 1).
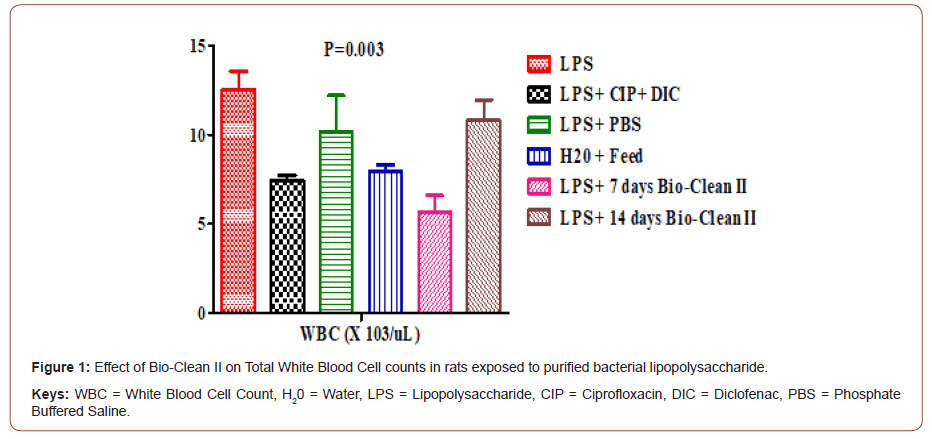
Effect on absolute lymphocytes Count: The effect of Bio- Clean II on LYM# counts in rats exposed to purified bacterial lipopolysaccharide is presented using a bar chart (Figure 2). The LYM# counts of 7days (4.04±0.85X103/μL) Bio-Clean II treated rat were found to be significantly lower (p= 0.004) when compared to the inflammation control group (7.66±0.75X103/μL); whereas, there was no significant difference in the LYM# of the 14days (7.68±0.87 X103/μL) Bio-Clean II treated rats. (p=1.000). Also, the LYM# of the 7days (4.04±0.85X103/uL) Bio-Clean II treated rat were found to be non-significantly lower (p=0.181) when compared to positive control group (4.36±0.61 X103/μL, p=1.000) whereas, the 14days (7.68±0.87 X103/μL) Bio-Clean II treated rats were found to be nonsignificantly higher (p=0.221). Still, the 7days (4.04±0.85X103/μL) Bio-Clean II treated rat were found to be non-significantly lower (p=0.181) when compared to the negative control (6.68±0.79X103/ μL, p=0.550), whereas, the 14days (7.68±0.87 X103/μL) Bio-Clean II treated rats were found to be significantly higher (p=1.000), also the LYM# count of the 7days (4.04±0.85X103/μL) Bio-Clean II treated rat were found to be non-significantly lower (p=0.969), whereas, 14days (7.68±0.87 X103/μL) Bio-Clean II treated rats were found to be non-significantly higher (p=0.640) when compared to Zero control group(5.44±0.52X103/μL). Furthermore, the LYM# count of 14days Bio-Clean II treated rats (7.68±0.87X103/μL) was found to be non-significantly higher (p=0.234) when compared to 7days Bio-clean II treated rats (4.04±0.85X103/μL). Meanwhile, the LYM# count of the Positive control (4.36±0.61X103/μL) was found to be non-significantly lower (p= 0.538) compared to the negative control group (6.68±0.79 X103/μL) (Figure 2).
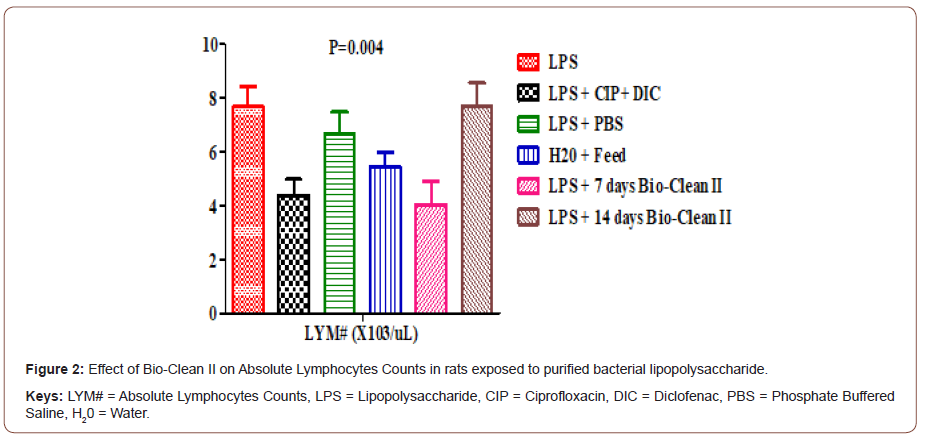
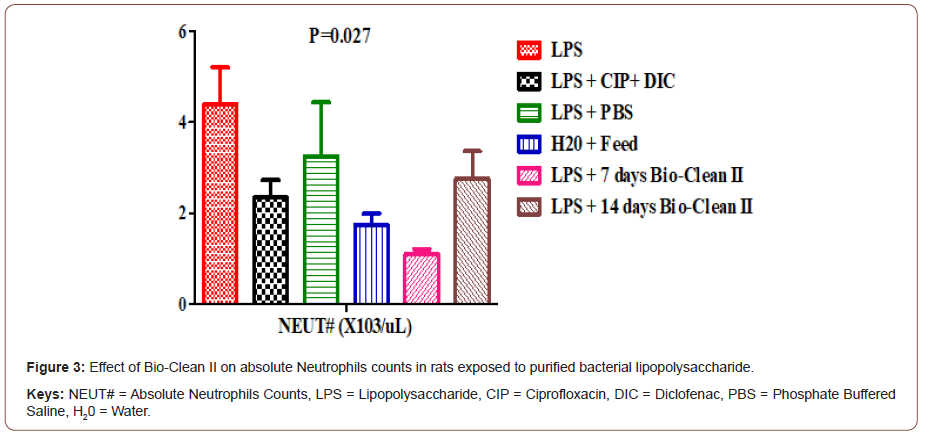
Effect on absolute neutrophils Count: The effect of Bio- Clean II on NEUT# counts in rats exposed to purified bacterial lipopolysaccharide is presented using a bar chart (Figure 3). The NEUT# count of 7days (1.10±0.11X103/μL) Bio-Clean II treated rat was found to be significantly lower (p= 0.027) when compared to the inflammation control group (4.40±0.81X103/μL). Also, the NEU# count of the 7days (1.10±0.11X103/μL) Bio-Clean II treated rats was found to be significantly lower (p= 0.029) when compared to the positive control group (2.36±0.37 X103/μL), while that of 14days (2.76±0.61 X103/μL) Bio- clean II treated rats was found to be non-significantly higher (p=1.000) when compared to the positive control, but on the other hand, that of the 7 days Bio-Clean II treated rats were found to be significantly lower (p=0.089), when compared to the negative control (3.26±1.18X103/μL). Furthermore, the NEUT# count of the 7days Bio-Clean II treated rats (1.10±0.11X103/μL) is non-significantly lower (p=0.617) when compared to the Zero control group (1.74±0.25 X103/μL), while 14 days Bio-Clean II treated rats (2.76±0.61X103/μL) was also observed to be non-significantly higher (p=0.948) when compared to the Zero control group (1.74±0.25 X103/μL). In addition, the NEUT# count level of 14 days Bio-Clean treated rats (2.76±0.61X103/μL) was found to be non-significantly higher (p=0.550) when compared to 7 days Bio-Clean II treated rats (1.10±0.11X103/μL). Meanwhile, the NEUT# count of the positive control (2.36±0.37X103/μL) was found to be non-significantly lower (p=1.000) than that of the negative control group (3.26±1.18 X103/μL) (Figure 3).
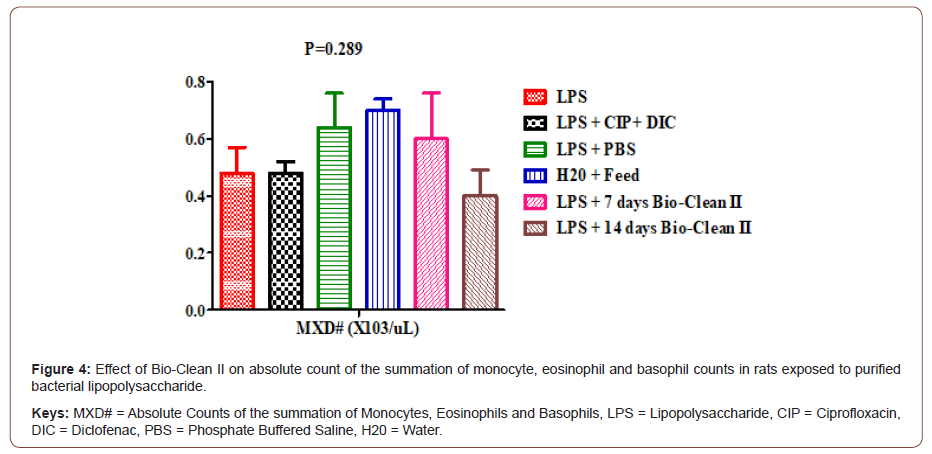
Effects on absolute counts of the summation of monocytes, eosinophils and basophils: The effect of Bio-Clean II on MXD# counts in rats exposed to purified bacterial lipopolysaccharide is presented using a bar chart (Figure 4). The MXD# count of 7days (0.60±0.16X103/μL) Bio-Clean II treated rats were found to be nonsignificantly higher (p=0.289) when compared to the inflammation control group (0.48±0.09X103/μL), while 14days (0.40±0.09X103/ μL) Bio-Clean II treated rat when compared to the inflammation control group (0.48±0.09X103/μL) to found to be non-significantly lower (p=1.000) when compared to. Also, on the other hand, the MXD# count of the 7days (0.60±0.16X103/μL) Bio-Clean II treated rats was found to be non-significantly higher (p=1.000) when compared to the positive control group (2.36±0.37 X103/uL), while that of the 14days (2.76±0.61 X103/μL) Bio- clean II treated rats was found to be non-significantly lower (p=1.000) when compared to the positive control (2.36±0.37 X103/μL), but on the other hand, the 7days Bio-Clean II treated rats (0.60±0.16X103/μL) was found to be non-significantly lower (p=1.000), when compared to the negative control (0.64±0.12X103/μL). Furthermore, the MXD# count of the 7days (0.60±0.16X103/μL) and 14days (0.40±0.09X103/μL) Bio- Clean II treated rats were non-significantly lower (p=1.000 and p=0.312, respectively) when compared to the Zero control group (0.70±0.04X103/μL). In addition, the MXD# count of 14 days Bio- Clean II treated rats (0.40±0.09X103/μL) was found to be nonsignificantly lower (p=0.997) when compared to 7days Bio-clean II treated rats (0.70±0.04 X103/μL). Meanwhile, the MXD# count of the positive control (0.48±0.04X103/μL) was found to be nonsignificantly lower (p=1.000) when compared to the negative control group (0.64±0.12 X103/μL) (Figure 4).
Discussion
This present study assessed the effects of Bio-Clean II on total and differential white blood cell count in rats exposed to purified bacterial LPS. In this study, the mean WBC increased significantly (P<0.05) in rats exposed to bacterial LPS only (inflammation control) compared to other groups. The inclusion of an inflammation control group in this study helps to confirm a successful induction of bacterial inflammatory disease in the experimental animals. It has been known that bacterial LPS are strong provoker of inflammation [5] and one of the immunological responses to inflammation is leukocytosis. According to Faas, et al. [26], when an animal is inoculated with bacterial LPS, leukocytes directionally migrate into inflammatory sites and secrete a large number of chemokine’s, adhesion factor, and pro-inflammatory cytokines to eliminate corresponding pathogens in a coordinated way. The outcome of this study disagrees with the works of Pham, et al. [27] and Kosumi, et al. [28] who both reported a remarkable decrease in white blood cells post exposure to LPS. Hounkpatin, et al. [29] also reported a significant decrease in WBC count postexposure to Cadium (12.50±1.20 X103/μL), Mercury (9.85±0.49 X103/μL) and combination of Cadmium and Mercury (8.15± 0.49 X103/μL) when compared to the control group (13.95±0.21 X103/ μL) administered normal saline. Furthermore, the outcome of this study contradicts the work of Al-Sagair, et al. [30], who investigated the effect of three types of bacterial LPS on bone marrow and blood components of rats. Group 1, 2 and 3 were injected intraperitoneally (1 mg/kg body weight) with single dose of LPS obtained from E. coli, K. pneumoniae and S. typhi, respectively and WBC count evaluated after 24hrs. The WBC count of the test rats decreased significant (p<0.001) after 24 hours of exposure to LPS of E. coli (3.46±0.1 X103/μL), K. pneumoniae (2.99±0.14 X103/μL) and S. typhimurium (2.83±0.0.17 X103/μL), in relative to the control (9.69±0.21 X103/μL) which received 0.9% normal saline. The WBC count of rats treated with Bio-Clean II for 7days in particular, was found to be significantly lower (p<0.05) when compared to that of the Inflammation control. This shows that the Bio-Clean II has the ability to reverse the leukocytosis associated with the inflammation induced by bacterial LPS. Still, the WBC count of rats treated with Bio-Clean II for 14 days was found to be non-significantly higher when compared to the Zero control group. This agrees with the work of Ajeigbe, et al. [31], who reported a significant (p<0.001) increase in the WBC of rats treated with 500mg/Kg (2.33±0.02 X 109Cells/l) aqueous leaf extract of Aspilia Africana compared to the Zero control (1.79±0.01 X109Cells/l). It should be noted that the Zero control rats did not receive LPS at all, but the Bio-Clean II treated rats did, thus explaining the non-significant increase in WBC count seen in this particular group of rats when compared to the Zero control group. In most clinical situation when a total white blood cell count is requested for, it is usually accompanying with a differential WBC count. This is to provide information on the proportion of the different white cells present in the circulating blood [32]. In this current study, the LYM# and NEUT# (except MXD#) of rats exposed to LPS was found to be significantly higher (P<0.05) in the inflammation control group compared to the Zero control group. Still, the LYM# and NEUT# of rats treated with Bio- Clean II for 7days were found to be non-significantly (p>0.05) lower when compared to the Inflammation control group, while the MXD# was found to be non-significantly higher than the inflammation control. This partly agrees with the work of Saba, et al. [33], who investigated the ameliorative potential of aqueous leaf extract of Cnidoscolus aconitifolius in rats exposed to Carbon tetrachloride (CCl4). On one hand, the absolute lymphocytes, neutrophils, monocytes and eosinophils count was found to be significantly (p<0.05) higher in the CCl4 induced group, when compared to the Zero control. But on the other hand, there was non-significant (p>0.05) decrease in the lymphocytes, neutrophils, monocytes and eosinophils count in rats treated with 750mg/kg aqueous leaf extract of Cnidoscolus aconitifolius, when compared to the CCl4 control. This shows that LPS alone, like CCl4 can induce lymphocytosis, neutrophilia, monocytosis, eosinophilia and basophilia in rats. On the other hand, 7 days Bio-Clean II treatment was capable of reversing lymphocytosis and neutrophilia, while the 14 days Bio-Clean treatment was capable of reversing monocytosis, eosinophilia and basophilia just like the 750mg/kg aqueous leaf extract of Cnidoscolus aconitifolius used by Saba, et al. [33]. This shows that Bio-Clean II must be administer for longer duration to achieve a reversal in monocytosis, eosinophilia, and basophilia as seen in the inflammation control. Furthermore, the outcome of this current study partly agrees and disagrees with the work of Al-Sagair, et al. [30], who investigated the influence of bacterial LPS on blood components. On one hand, the absolute lymphocyte, monocyte and eosinophil count evaluated after 24hrs decreased significantly (p<0.001) in animals injected with E. coli (852.00±40.49μL, 144.00±19.4μL and 324.00±29.59μL, respectively), K. pneumoniae (799.00±52.79μL, 132.00±20.79μL and 183.00±24.86μL, respectively) and S. typhimurium (568.00±53.54μL, 87.00±30.29μL and 178.00±25.21μL, respectively) LPS, when compared to the control group (6078.00±310.72μL, 644.00±40.74μL and 346.00±35.41μL, respectively) which received 0.9% normal saline. But on the other hand, the absolute neutrophil count increased significantly (p<0.001) in animals injected with E. coli (342.00±27.56μL), K. pneumoniae (270.00±19.49μL) and S. typhimurium (306.00±29.6μL) LPS when compared to the control group (255.00±21.31μL). The reason for these differences is plausible, but it is not unlikely to be connected to the immunogenicity of the animals used and the type of LPS tested. In this study, animals in the Zero control group were given only feed and water (no LPS), while the Bio-Clean treated rats were first induced with LPS, before been treated afterwards. The lack of LPS induction in the Zero control explains the lower LYM# and NEUT# observed in this study, when compared to the 14 days Bio-Clean II treated groups. This is in agreement with the work of Ajeigbe, et al. [31], who reported a higher absolute Neutrophils and Absolute Lymphocytes counts post administration with the 500mg/Kg aqueous leaf extract of Aspillia africana (1.32±0.04 X 109Cells/l; 1.43±0.05 X 109Cells/l, respectively) when compared to the Zero control (0.80±0.00 X 109Cells/l; 0.83±0.00 X 109Cells/l), p<0.05 and p<0.001, respectively. The increase observed in the neutrophil and the lymphocytes count in this study post-induction with LPS was justified since these immune cells respond primarily to infection caused by bacteria and bacterial products such as LPS. However, the reversal of the leukocytosis as observed in the group treated with Bio-Clean II for 7 days can be attributed to the ameliorative and immunomodulating potential of the herbal remedy. The presence of some metabolic components with antioxidant and antiinflammatory properties may be responsible for these effects. This further gives credence to the health benefits of the herbal remedy as previously reported [17-19].
Conclusion
Bio-Clean II is a promising immunomodulating agent in the treatment of bacterial inflammatory diseases as it reversed the leukocytosis associated with exposure to purified bacteria lipopolysaccharide, further confirming the inherent antiinflammatory property of the herbal remedy.
Ethical Approval
Ethical approval for the study was obtained from the Babcock University Health Research Ethics Committee (BUHREC) with ethical approval registration number: BUHREC 509/21.
Acknowledgement
None.
Conflict of Interest
The authors report no conflict of interest.
References
- Terzić M, Kocijančić D (2010) Pelvic inflammatory disease: contemporary diagnostic and therapeutic approach. Srpski arhiv za celokupno lekarstvo 138(9-10): 658-663.
- Janeway CA, Travers P, Walport M, Shlomchik M (1996) Immunobiology: the immune system in health and disease. London: Current Biology 7: 26.
- Levi M, Keller TT, Van-Gorp E, Ten-Cate H (2003) Infection and inflammation and the coagulation system. Cardiovascular Research 60(1): 26-39.
- Park BS, Song DH, Kim HM, Choi BS, Lee H, et al. (2009) The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 Nature 458(7242): 1191-1195.
- Mayer H, Tharanathan RN, Weckesser J (1985) Analysis of Lipopolysaccharides of Gram-negative bacteria. Meth Microbiol 18: 157-207.
- Bell MJ, Hallenbeck JM (2002) Effects of intrauterine inflammation on developing rat brain. Journal of neuroscience research 70(4): 570-579.
- Dantzer R (2004) Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. European journal of pharmacology 500(1-3): 399-411.
- Kussmann M (2010) Nutrition and Immunity (Chapter 9). RSC Food Analysis Monographs No. 9 Mass Spectrometry and Nutrition Research Edited by Laurent B Fay, Martin Kussmann. The Royal Society of Chemistry 2010 Published by the Royal Society of Chemistry, pp. 268-309.
- Curry A, Williams T, Penny ML (2019) Pelvic inflammatory disease: diagnosis, management, and prevention. American family physician 100(6): 357-364.
- Nathan C (2006) Neutrophils and immunity: challenges and opportunities. Nature reviews immunology 6(3): 173-182.
- Stramer BM, Mori R, Martin P (2007) The inflammation–fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. Journal of Investigative Dermatology 127(5): 1009-1017.
- Fujiwara N, Kobayashi K (2005) Macrophages in inflammation. Current Drug Targets-Inflammation and Allergy 4(3): 281-286.
- Jabbour HN, Sales KJ, Catalano RD, Norman JE (2009) Inflammatory pathways in female reproductive health and disease. Reproduction 138(6): 903-919.
- World Health Organization (WHO) (2008) Herbal Medicine. World Health Organization, Geneva.
- Ibeh IN, Akanu N, Mkpa AM, Isitua CC, Ogefere HO, et al. (2013) Evaluation of the Anti-Human Immunodeficiency Virus (HIV) Properties of DXL (Decoction X-Liquid-Bioclean II). J Clin Toxicol 12(4): 1-7.
- Ibeh IN, Okungbowa MA, Ibeh NI, Adejumo BI (2016a) Comparative studies on the effects of Zidovudine + Nevirapine + Lamivudine and Bio-clean II on female HIV/AIDS cases in Nigeria. JAMPS 9(3): 1-7.
- Enitan SS, Ibeh IN, Akele RY, Isitua CC, Jegede OO, et al. (2022a) Immunomodulatory Effects of Bio-Clean II on some Inflammatory Cytokines in Rats Exposed to Purified Bacterial Lipopolysaccharide. Immunology and Inflammation Diseases Therapy.
- Enitan SS, Ibeh IN, Adelakun A, Akele RY, Olayanju AO, et al. (2022b) Immunomodulatory Effects of Bio-Clean II Herbal Remedy on C-reactive protein, Corticosterone and Antiphospholipid antibodies in Rats exposed to Purified Bacterial Lipopolysaccharide. Journal of Clinical and Experimental Immunology.
- Enitan SS, Ibeh IN, Nwaenyi KE, Adelakun A, Akele RY, et al. (2022c) Immunomodulatory Effects of Bio-Clean II on T-Helper 4 and Cytotoxic T-Lymphocytes in Rats exposed to Purified Bacterial Lipopolysaccharide. Annals of Immunology and Immunotherapy 4(1): 1-9.
- Ibeh IN, Okungbowa MA, Ibeh NI, Isitua CC (2016b) Studies on HIV Resistance to Multiple Drug Therapy (Lamivudine, Zidovudine and Nevirapine) in Benin Metropolis, Nigeria. International Journal of Scientific Research in Environmental Science and Toxicology 1(1): 1-3.
- Enitan SS, Ibeh IN, Akele RY, Isitua CC, Idris PO (2022d) Phytochemical Screening, Trace Element Analysis, Bacteriological Quality and Efficacy of Bio-Clean II Herbal Remedy. Traditional Medicine and Modern Medicine.
- ILAR (1996) Guide for the care and use of laboratory animals. Institute for Laboratory Animal Research Council Division on Earth and Life Studies American Academy of Sciences National Research Council of the National Academies the National Academies Press 500 Fifth Street NW Washington 11(8): 131.
- Ochei J, Kolhatkar A (2006) Euthanasia of Animals by Cervical Dislocation. In: Ochei J, Kolhatkar A (), Theory and Practice of Medical Laboratory Science. Tata McGraw-Hill publishing Company Limited, New Delhi: 1213-1230.
- Samuel OI, Thomas N, Ernest OU, Imelda NN, Elvis NS, et al. (2010) Comparison of haematological parameters determined by the sysmex KX-21N automated haematology analyzer and the manual counts. BMC Clinical Pathology 10: 3.
- Shott S (1990) Statistics for health professionals. Saunders WB Co Philadelphia, pp. 313-336.
- Faas MM, Moes H, Van der Schaaf G, De Leij LF, Heineman MJ (2003) Total white blood cell counts and LPS-induced TNFα production by monocytes of pregnant, pseudopregnant and cyclic rats. Journal of reproductive immunology 59(1): 39-52.
- Pham D, Jeng AY, Escher E, Sirois P, Battistini B (2000) Effects of a selective neutral endopeptidase and a nonselective neutral endopeptidase/endothelin-converting enzyme inhibitor on lipopolysaccharide-induced endotoxaemia in anaesthetized Sprague-Dawley rats. Journal of cardiovascular pharmacology 36(5): 362-366.
- Kosumi T, Usui N, Kubota A, Hoki H, Yamauchi K, et al. (2001) Application of a drug delivery system in a novel rat model of chronic hyperendotoxemia. Pediatric surgery international 17(4): 321-325.
- Hounkpatin ASY, Edorh PA, Guédénon P, Alimba CG, Ogunkanmi A, et al. (2013) Haematological evaluation of Wistar rats exposed to chronic doses of cadmium, mercury and combined cadmium and mercury. African Journal of Biotechnology 12(23): 3731-3737.
- Al-Sagair OA, El-Daly ES, Mousa AA (2009) Influence of Bacterial Endotoxins on Bone Marrow and Blood Components. Medical Journal of Islamic World Academy of Sciences 17(1): 23-36.
- Ajeigbe KO, Enitan SS, Omotoso DR, Oladokun OO (2013) Acute effects of aqueous leaf extract of Aspilia africana CD Adams on some haematological parameters in rats. Afr J Tradit Complement Altern Med 10(5): 236-243.
- Tatfeng YM, Enitan, SS (2012) Effects of Allium sativum and Allium cepa on some Immunological Cells in Rats. African Journal of Traditional, Complementary and Alternative Medicine 9(3): 374-379.
- Saba AB, Oyagbemi AA, Azeez OI (2010) Amelioration of carbon tetrachloride-induced hepatotoxicity and haemotoxicity by aqueous leaf extract of Cnidoscolus aconitifolius in rats. Nigerian Journal of Physiological Sciences 25(2): 139-147.
-
Seyi Samson Enitan, Isaiah Nnanna Ibeh, Ayodeji Olusola Olayanju, Emmanuel Olusegun Ileoma, Abiodun Durosinmi, et al. Immunomodulatory Effects of Bio-Clean II on Total and Differential Leucocyte Counts in Rats Exposed to Purified Bacterial Lipopolysaccharide. Open J Pathol Toxicol Res. 1(2): 2021. OJPTR.MS.ID.000509.
-
Gram-negative bacteria, Toll-like receptor-4, Cytokines, Chemokines, Chronic inflammatory diseases, Atherosclerosis, Diabetes, Autoimmune, Degenerative diseases, Asthma, Cancer, C-reactive protein, Corticosterone, Antiphospholipid antibodies
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






