 Research Article
Research Article
Prospective, Monocentric, Repeated-Measures Study of ADHEAR in Adults with Middle Ear Disease
Rami Abu Dakah1, Dörte Fischer1,2 and Tino Just1*
1Department of Otorhinolaryngology, Head and Neck Surgery, KMG Klinikum, Güstrow, Germany,
2Department of Audiology, KMG Klinikum, Güstrow, Germany
Professor Tino Just (ORCID-ID: 0000-0003-2036-8569)
Dr. Dörte Fischer (ORCID-ID: 0000-0003-3736-4441)
Tino Just, M.D., Department of Otorhinolaryngology, Head and Neck Surgery Friedrich-Trendelenburg-Allee 1, D-18273 Güstrow, Germany.
Received Date:February 16, 2024; Published Date:February 27, 2024
Abstract
Objective: The aim of this study was to evaluate the audiological performance of the ADHEAR system and to compare it with a softband BAHA
system in adults with middle ear disease.
Study Design: Prospective, single-subject, repeated-measures study
Setting: Monocentric study
Participants: In 23 patients, with mild to moderate conductive hearing loss, the ADHEAR system was tested, where 8 of them were outside of
the ADHEAR indication. In ten patients, testing was performed with the ADHEAR system and a softband BAHA system. A control group consisted
of 10 patients with simulated bilateral conductive hearing loss.
Main outcome measures: Air and bone conduction thresholds and free-field monosyllable speech intelligibility thresholds in quiet and in
noise were measured in unaided and aided situation with the ADHEAR and a softband BCHD. Furthermore, ADHEAR questionnaire was assessed.
Results: In patients with a middle ear disease compared to the unaided situation, the aided situations with the ADHEAR system and the
softband BAHA system led to a significant improvement of speech intelligibility in quiet and in noise. Outside the device indication performance
was limited. In terms of the ADHEAR questionnaire, using the ADHEAR system patients reported an improvement in sound quality and speech
intelligibility.
Conclusion: Due to the pressure-less wearing comfort we see the ADHEAR system as a treatment option in patients who are unable to receive
or deny surgery. Outside of its indication use must be evaluated individually. Softband BAHA systems remain an alternative solution for patients
with conductive hearing loss.
Keywords:Adhear; Bone conduction device; adhesive adapter; Baha
An earlier version of the manuscript has been presented as reprint in Authorea.
Five succinct key points.
1. The ADHEAR leads to similar hearing outcomes as a softband BAHA system in patients with conductive hearing loss.
2. The benefit with the ADHEAR for patients with middle ear disease outside of ADHEAR indication is limited and must be trialed individually.
3. The pressure less wearing comfort of this adhesive hearing device can be a permanent or temporary treatment option in patients who are
unable to receive or deny middle ear surgery.
4. For patients with middle ear disease, ADHEAR can be a treatment option between the diagnosis and surgery or a hearing aid supply.
5. Softband BAHA systems remain an alternative solution for patients with conductive hearing loss.
Introduction
For about four decades, bone conduction hearing devices (BCHDs) used to improve hearing in patients with single-sided deafness (SSD) and conductive or mixed hearing loss (CHL or MHL) [1]. Through vibration of the skull or structures of the middle ear, these devices transmit signals directly to the cochlea.
BCHDs can be divided into implantable and non-implantable devices. While implantable devices consist of an implant and an audio processor (AP), non-implantable devices consist of an AP and an adapter for non-invasive AP retention on the head [2, 3].
Implantable BCHDs are the active percutaneous or passive transcutaneous bone-anchored hearing aids (BAHAs), the Ponto™ system (Oticon Medical, Denmark) and the BAHA® Attract or Connect systems (Cochlear, Sweden) [4]. Active transcutaneous systems are the Osia™ (Cochlear, Sweden) [5] or the Bonebridge® (Med-el, Innsbruck, Austria) [6]. The only active transcutaneous middle ear implant system is the Vibrant Soundbridge® (Med-el, Innsbruck, Austria) [7].
As a non-implantable option, in 2001 a softband adapter for AP was introduced, especially for children. Many studies show that the softband BCHD is a successful treatment for CHL [8-10]. However, due to the static pressure on the head, some children and adults do not tolerate a softband as a long-term solution [11]. The ADHEAR was introduced in 2017, as a pressure-free non-implantable BCHD [12-16]. The key concept of the ADHEAR system is a disposable adhesive adapter sticking to the hairless skin behind the ear, with an AP clicking onto this adapter. Previous studies show that the audiometric results using the ADHEAR system are similar to those using a softband BCHD system [16], while the wearing time and acceptance in children and adults is preferred over a softband BAHA system [12, 16].
Objective
The aim for this study was to re-evaluate if adults with chronic middle ear disease of various etiologies outside the indication criteria may benefit from the ADHEAR system, regardless of its recommended indication criterion.
Methods
Ethical considerations
This study was conducted in strict adherence to the revised version of the Helsinki Declaration. The design of the study was approved by the Ethics Committee of the General Medical Council of Mecklenburg-West Pomerania (A2018-0035).
Participants
For participation, the inclusion criteria were German native speakers aged between 18 to 80 years with middle ear disease resulting in a CHL or MHL, unilateral or asymmetric hearing loss (HL). Patients with ear surgery of the contralateral ear, retrocochlear or central auditory disorders, skin diseases or other surgical conditions that may prevent the attachment of the adhesive adapter of the ADHEAR system were excluded.
Overall, 23 patients of the Department of Otorhinolaryngology, Head and Neck Surgery with middle ear disease (middle ear group) and 10 healthy volunteers as a control group participated in this prospective single-subject repeated-measure study (Table 1). All subjects were informed about the aims of the study and provided their written consent.
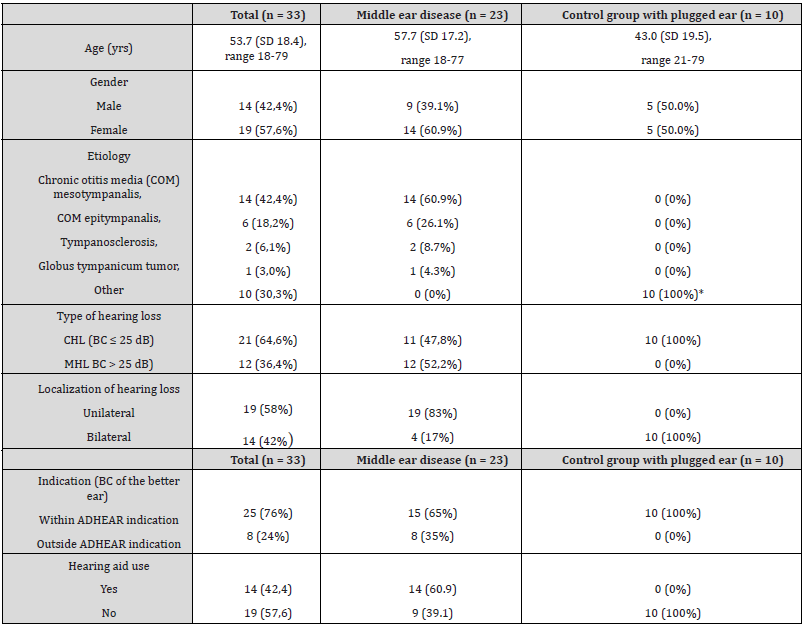
Table 1:Demographics and baseline characteristics for all participants, for the subgroup of patients with middle ear diseases and for the control group (subjects with bilateral plugged ears).
Procedure
In all patients, testing was performed with the ADHEAR system and in ten additionally a softband BAHA system.
All patients and healthy subjects obtained a detailed medical report and an ear, nose and throat examination. The patients with middle ear disease were identified by otoscopy. In all healthy subjects, a chronic otitis media was excluded.
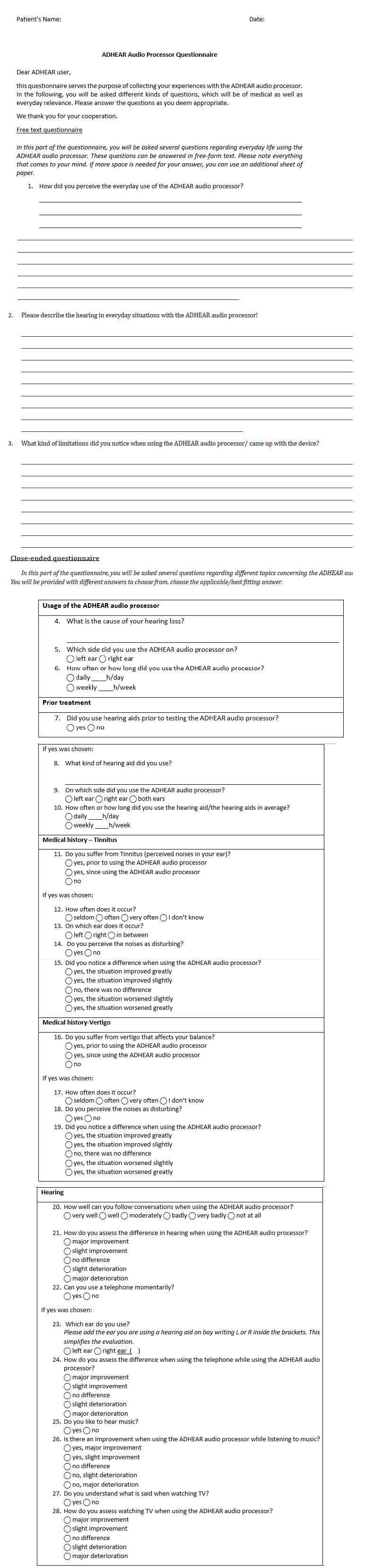
All participants obtained a pure tone audiometry and free-field speech audiometry under various conditions, i.e., in the unaided and aided situations. All subjects used the ADHEAR system. Due to the reduced number of BAHA Power 5 systems, in only ten subjects both devices ADHEAR and softband BAHA 5 Power system were tested. The patient’s selection for testing both systems was not made at random. All patients were invited to be evaluated for both systems. The patients did self-select themselves. Furthermore, all patients were asked to fill out the custom made ADHEAR questionnaire (supplementary material, Table S1).
Table S1:Questionnaire
Outcome measures
All audiological assessments were conducted in an audiometric sound attenuated room, using calibrated signals and equipment according to accepted ISO standard. For assignment of the participants a pure tone audiometry was performed measuring air conduction (AC) and bone conduction (BC) thresholds. The PTA4 was calculated across the frequencies of 0.5, 1, 2, and 4 kHz.
The speech intelligibility tests were conducted in the free field using the Freiburger monosyllables word test in quiet and in noise. The loudspeakers were positioned 1m away from the participants’ head. In quiet, i.e., under the S0 condition, the speech intelligibility was measured at 65 dB SPL. In noise, i.e., under the S0N0 condition, the speech intelligibility was measured with a fixed noise level at 60 dB SPL and a speech level at 65 dB SPL, resulting in a signal-tonoise ratio (SNR) of 5 dB. All speech intelligibility measurements were performed in the unaided situation and the aided situation with the ADHEAR system. In order to compare the performance of the ADHEAR system, 10 patients and the 10 healthy subjects were also fitted with a softband BAHA system using the BAHA 5 Power system. In combination with the Cochlear BAHA, fitting software (Cochlear Fitting Suite 1.8) was used according to the patient’s audiometric hearing thresholds.
In order to measure the speech intelligibility of the ipsilateral ear as isolated as possible, the contralateral ear was occluded using an earplug (Oropax®). No masking of the bone conduction of the better hearing contralateral ear was performed. For analysis, the BC of the better hearing ear was used. In the control group, the healthy subjects with normal or age-appropriate hearing ability, both ears were plugged with an earplug (Oropax®) reaching 40±8.2 dB HL (range 22.5-50 dB HL). A questionnaire focused on several aspects encountered in applying the ADHEAR system, namely handling of the system, hearing sensation and problems (supplementary material, Table S1).
Data analyses
Statistical tests (Kolmogorov Smirnov test and Shapiro- Wilks test) indicated non-normal distribution for all parameters. Nonparametric tests were performed for group comparisons whenever appropriate. Pearson statistics were used for correlational analyses. The alpha level was set to 0.05. SPSS 27.0 (SPSS Inc. Chicago, IL, USA) was used for statistical analyses.
Results
Middle Ear Group
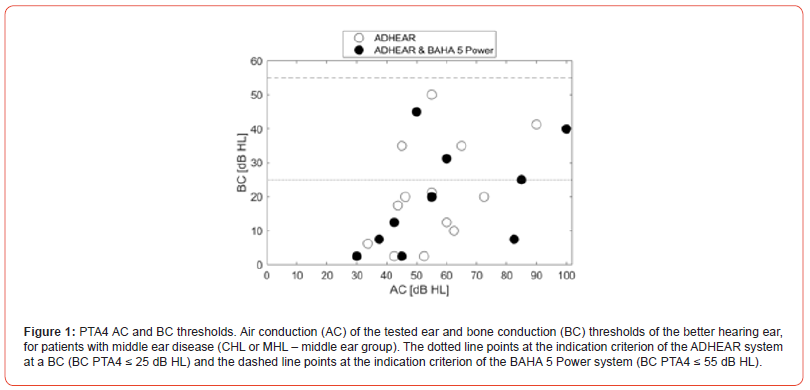
23 patients with middle ear disease (19 unilateral, 4 bilateral) age range from 18 to 77 (mean 58 years of age), 14 females and 9 males were enrolled. The measured PTA4 AC values of the tested ear and the BC values of the better hearing ear are presented in Figure 1. The dotted and the dashed lines present the indication criteria for the ADHEAR system (BC PTA4 ≤ 25 dB HL) and for the BAHA 5 Power system (BC PTA4 ≤ 55 dB HL), respectively. For analysis, the BC of the better hearing ear was used. Figure 1 shows that 23 patients were aided with the ADHEAR system, while the HL of only 15 patients is within the ADHEAR criterion. Ten patients were aided with both the ADHEAR and the BAHA 5 Power system, while the HL of 6 of these 10 patients is within the ADHEAR criterion.
Pure Tone Audiometry
The mean BC threshold was 20 ± 15 dB HL and the air bone gap
on the tested side was 29 ± 14 dB HL. On the contralateral side, a
mean air bone gap of 9.5 ± 11 dB HL was found.
Speech Audiometry in Quiet and in Noise
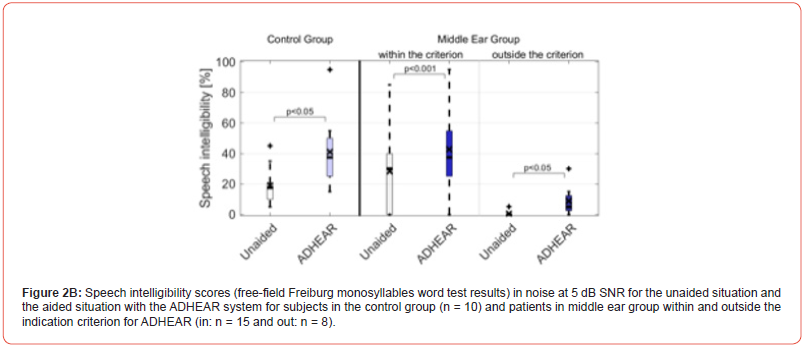
Using the Freiburg monosyllables word test, the mean speech
intelligibility score in quiet at 65 dB SPL in the unaided situation
(45.3% ± 38.8%) significantly improved to 68.0% ± 26.4% using
the ADHEAR system (p < 0.01), in patients within the ADHEAR
indication criterion (BC PTA4 ≤ 25 dB HL). In patients outside that
ADHEAR indication criterion, the word recognition score in the
unaided situation (10.0% ± 12.8%) improved to 20.6% ± 15.7%
using the ADHEAR system (p = 0.073).
At 5 dB SNR, the speech intelligibility score in the unaided situation (26.3% ± 24.7%) significantly improved to 42.9% ± 27.5% using the ADHEAR system (p < 0.001), in patients within the ADHEAR indication criterion. For patients outside the criterion, the speech intelligibility score in the unaided situation (0.62% ± 1.77%) significantly improved to 8.75% ± 9.91% using the ADHEAR system (p < 0.05).
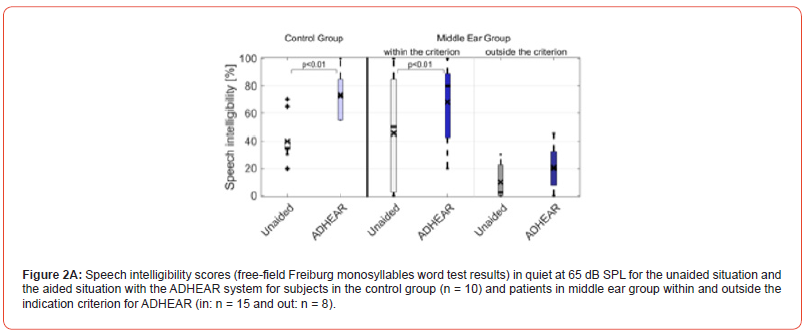
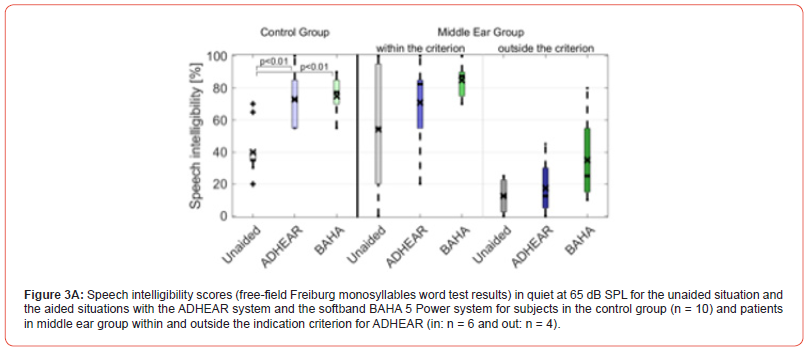
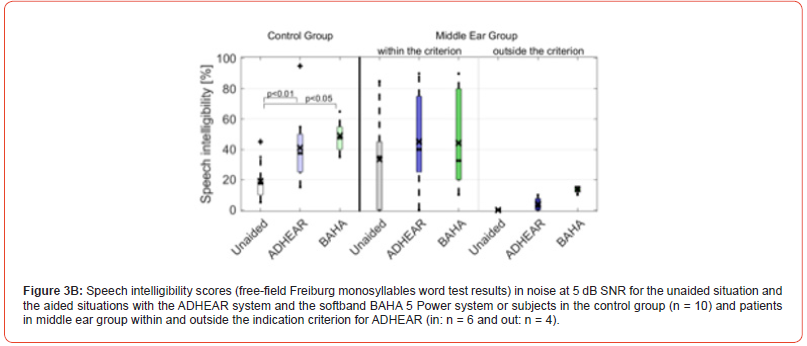
Considering ADHEAR criterion, Figures 2A and B depict the speech intelligibility scores of the 23 middle ear patients (15 patient within ADHEAR criterion and 8 patients outside ADHEAR criterion) for the unaided situation and the aided situation with ADHEAR in quiet at 65 dB SPL and in noise at 5 dB SNR, respectively. Figures 3A and B depict the speech intelligibility scores of the 10 out of 23 patients with middle ear disease trialed both ADHEAR and BAHA 5 Power (see Figure 1) and were tested in quiet and in noise, respectively.
Control Group (Healthy Subjects)
In terms of the Freiburg monosyllables word test in quiet at 65 dB SPL, the mean speech intelligibility score in the unaided situation (40.0% ± 15.5%) was significantly improved to 75% ± 11.3% using the BAHA 5 Power system (p < 0,01) and to 73.0 ± 15.9% using the ADHEAR system (p < 0.01).
At 5 dB SNR, the speech intelligibility scores in the unaided situation decreased from 40.0% ± 15.5% in quiet to 19.0 ± 12.4%. The mean speech intelligibility score in the unaided situation was significantly improved to 49.0% ± 9.7% using the BAHA 5 Power system (p < 0,01), and to 41.0 ± 23.2% using the ADHEAR system (p < 0.05).
Table 2:Mean speech intelligibility results between the unaided situation and the aided situations, using the ADHEAR system and a softband BAHA 5 Power system.
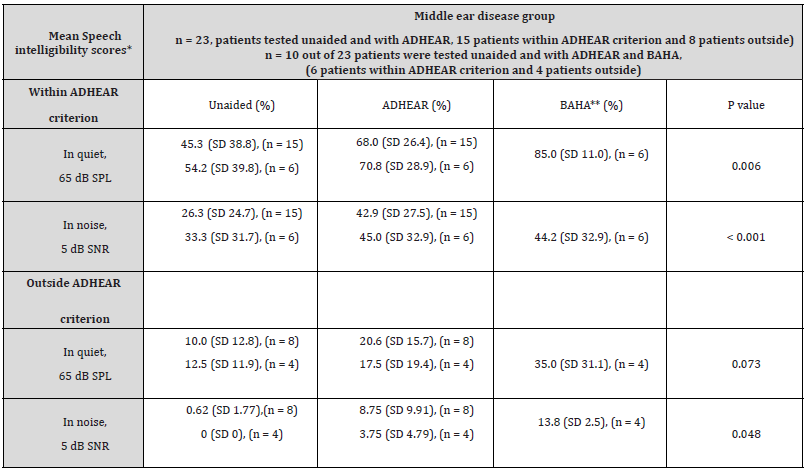
*Speech intelligibility test-Freiburg monosyllable word test in quiet and in noise, at a speech level of 65 dB SPL, and a fixed noise level of 60 dB SPL
resulting in a signal-to-noise ratio of 5 dB.
**Softband BAHA 5 Power
Questionnaire
The answer categories to the free-text questions are presented in Tables S2-4 (supplementary material, Tables S2-4). Main aspects are use of AP, improvement of sound quality and speech perception with ADHEAR compared to the unaided situation. In three patients of the middle ear group and in two subjects of the control group problems with the adhesive adapter over time was reported. However, the majority reported on easy use and improved spatial hearing.
Table S2:Categories identified to the free-text question: “Please describe the hearing in everyday situations with the ADHEAR audio processor!”.
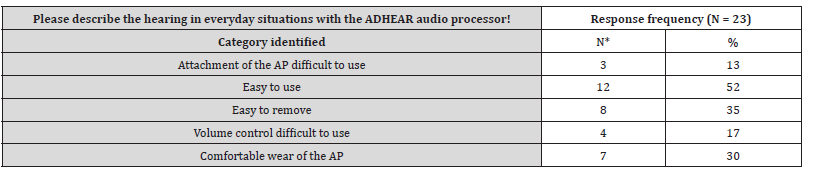
*Double counting possible.
Table S3:Categories identified to the free-text question: “How do you assess the difference in hearing when using the ADHEAR audio processor?”.
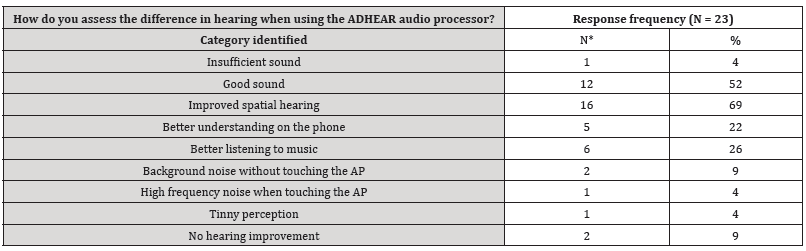
*Double counting possible.
Table S4:Categories identified to the free-text question: “What kind of limitations did you notice when using the ADHEAR audio processor/came up with the device?”.
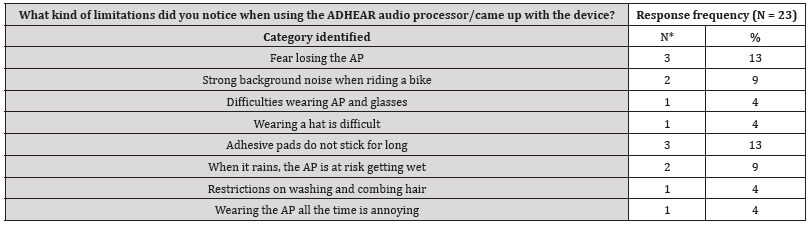
*Double counting possible.
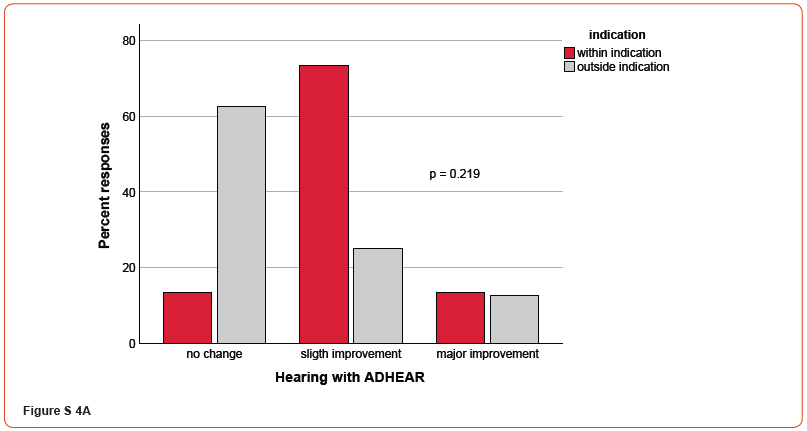
Figures 4A-C demonstrate the patient’s response to questions relating to hearing in general (A), speech perception (B), and music perception (C), respectively (supplementary material, Figures S4). For all responses, no significant differences were found regarding the ADHEAR indication (all p’s > 0.05). Additionally, correlational analyses revealed no significant correlations between free-field monosyllable speech intelligibility thresholds at 65 dB in quiet with ADHEAR and assessment of hearing, speech perception over telephone and music perception (all p’s > 0.05), respectively.
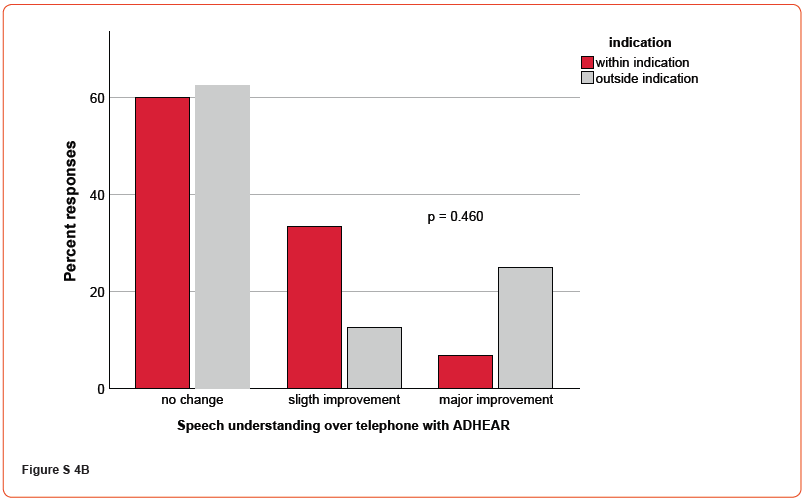
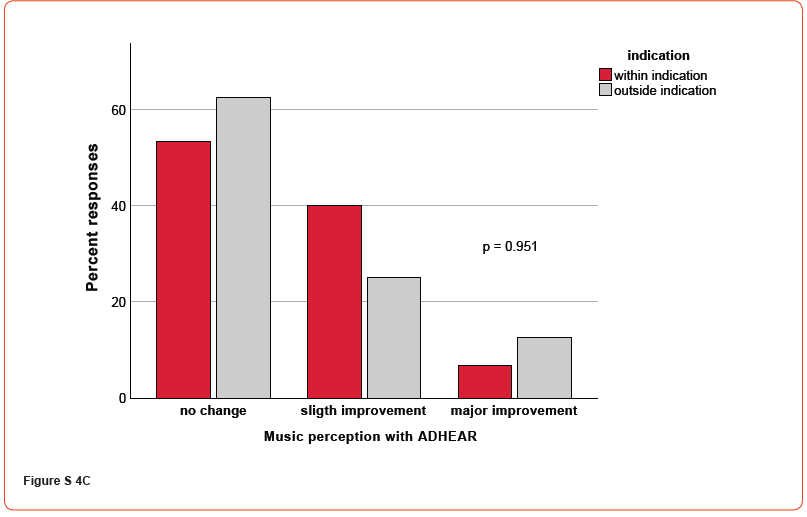
Discussion
In our study the ADHEAR system was applied to an inhomogeneous group of patients with middle ear diseases of multiple etiologies suffering from a uni-or bilateral CHL or MHL. In contrast to previous studies, ADHEAR was also applied to patients whose BC of the better hearing ear was outside the indication criterion. A limitation of this study is the small number of patients.
Comparing the results within (BC ≤ PTA4 25 dB HL) and outside (BC > PTA4 25 dB HL) the ADHEAR criterion, the performance of both the ADHEAR and BAHA 5 Power clearly reduced. However, if there are no treatment alternatives, the small improvements in speech intelligibility and assumingly even higher benefit in general sound perception, might be still valuable for the patient.
Within the criterion, clinically relevant improvement in speech intelligibility in quiet and noise was measured using the softband BAHA or the ADHEAR system compared to the unaided situation. Similar results were found in a pilot study by Skarzynski, et al. [17].
Another study on 10 patients after middle ear surgery showed that in comparison to the unaided situation, the ADHEAR leads to a significant speech intelligibility improvement of monosyllables in quiet at 65 dB [18]. The authors concluded that this adhesive BCHD may considerably improve treatment of patients with even shortterm HL.
In patients with chronic inflammation of the outer ear canal and/or the middle ear, a conventional hearing aid is often refused by the doctor during conservative treatment. Even after middle ear surgery or surgery of the outer ear canal there is also a need for a temporary alternative for hearing improvement. In these cases, a softband BAHA system or an adhesive BCHD may be an alternative to improve hearing.
A softband BAHA system is sometimes refused by the patients due to social stigmatization and sweating, especially in summer. Therefore, we initialized the study starting in summer to prove whether the ADHEAR system is an alternative to a softband BAHA system or not.
According to the company information, the adhesive adapter is designed for single use and can remain on the skin for 3-7 days [9, 12, 15, 19]. Considering the statements in our questionnaire, in five out of 32 participants of our study, the adhesive adapter detached due to sweating. The reported daily use and the comfort level was high, suggesting good patient’s acceptance. Additionally, no allergic reactions or other skin alteration were reported. However, for a few patients the adhesive solution was not satisfactory such that the softband BAHA remained an alternative.
Patients with long-time experience for the ADHEAR and the softband BAHA system reported a higher wearing comfort and longer wearing time with the ADHEAR system than the softband BAHA system [16, 19]. More data are needed to conclude that an adhesive solution is the preferred option.
Conclusion
Our study results allow the conclusion that the decision which modality can be recommended to improve hearing in patients with middle ear disease is not only audiologic but involves both audiological and clinical assessment. Due to the pressure-less wearing comfort we see the ADHEAR system as a permanent or temporary treatment option in patients who are unable to receive or deny surgery. Softband BAHA systems remain a solution for patients were the adhesive wearing time is not satisfactory.
Declaration of Funding Sources or Other Conflicts of Interest
The authors received no financial support for the research authorship and/or publication of this article.
Data Sharing and Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Authorship Statement Using ICJME Criteria and Author Initials
Tino Just and Rami Abu Dakah designed the work; Rami Abu Dakah acquired data. Dörte Fischer and Tino Just analyzed data. Tino Just, Dörte Fischer and Rami Abu Dakah drafted, revised and approved the manuscript; Tino Just and Dörte Fischer agree to be accountable for all aspects of the work.
References
- Tjellstrom A, Hakansson B, Granstrom G (2001) Bone-anchored hearing aids: current status in adults and children. Otolaryngol Clin North Am 34(2): 337-364.
- Cedars E, Chan D, Lao A, Hardies L, Meyer A, et al. (2016) Conversion of traditional osseointegrated bone-anchored hearing aids to the Baha((R)) attract in four pediatric patients. Int J Pediatr Otorhinolaryngol 91: 37-42.
- Gawecki W, Stieler OM, Balcerowiak A, Komar D, Gibasiewicz R, et al. (2016) Surgical, functional and audiological evaluation of new Baha((R)) Attract system implantations. Eur Arch Otorhinolaryngol. 273(10): 3123-3130.
- Oberlies NR, Castano JE, Freiser ME, McCoy JL, Shaffer AD, et al. (2020) Outcomes of BAHA connect vs BAHA attract in pediatric patients. Int J Pediatr Otorhinolaryngol 135: 110125.
- Mylanus EAM, Hua H, Wigren S, Arndt S, Skarzynski PH, et al. (2020) Multicenter Clinical Investigation of a New Active Osseointegrated Steady-State Implant System. Otol Neurotol 41(9): 1249-1257.
- Sprinzl G, Lenarz T, Ernst A, Hagen R, Wolf Magele A, et al. (2013) First European multicenter results with a new transcutaneous bone conduction hearing implant system: short-term safety and efficacy. Otol Neurotol 34(6): 1076-1083.
- Labassi S, Beliaeff M, Pean V, Van de Heyning P (2017) The Vibrant Soundbridge® middle ear implant: A historical overview. Cochlear Implants Int 18(6): 314-323.
- Hol MK, Cremers CW, Coppens Schellekens W, Snik AF (2005) The BAHA Softband. A new treatment for young children with bilateral congenital aural atresia. International Journal of Pediatric Otorhinolaryngology 69(7): 973-980.
- Osborne MS, Child-Hymas A, McDermott AL (2020) Longitudinal study of use of the pressure free, adhesive bone conducting hearing system in children at a tertiary centre. Int J Pediatr Otorhinolaryngol 138: 110307.
- Verhagen CV, Hol MK, Coppens-Schellekens W, Snik AF, Cremers CW (2008) The Baha Softband. A new treatment for young children with bilateral congenital aural atresia. Int J Pediatr Otorhinolaryngol 72(10): 1455-1499.
- Reinfeldt S, Hakansson B, Taghavi H, Eeg Olofsson M (2015) New developments in bone-conduction hearing implants: a review. Med Devices (Auckl) 8: 79-93.
- Dahm V, Baumgartner WD, Liepins R, Arnoldner C, Riss D (2018) First Results With a New, Pressure-free, Adhesive Bone Conduction Hearing Aid. Otol Neurotol 39(6): 748-754.
- Dobrev I, Farahmandi TS, Huber AM, Roosli C (2021) Experimental Evaluation of the Adhear, a Novel Transcutaneous Bone Conduction Hearing Aid. Laryngorhinootologie 100(10): 811-817.
- Favoreel A, Heuninck E, Mansbach AL (2020) Audiological benefit and subjective satisfaction of children with the ADHEAR audio processor and adhesive adapter. Int J Pediatr Otorhinolaryngol 129: 109729.
- Hirth D, Weiss R, Stöver T, Kramer S (2021) Audiological benefit and subjective satisfaction with the ADHEAR hearing system in children with unilateral conductive hearing loss. Eur Arch Otorhinolaryngol 278(8): 2781-2788.
- Neumann K, Thomas JP, Voelter C, Dazert S (2019) A new adhesive bone conduction hearing system effectively treats conductive hearing loss in children. Int J Pediatr Otorhinolaryngol 122: 117-125.
- Skarzynski PH, Ratuszniak A, Osinska K, Koziel M, Krol B, et al. (2019) A Comparative Study of a Novel Adhesive Bone Conduction Device and Conventional Treatment Options for Conductive Hearing Loss. Otol Neurotol 40(7): 858-864.
- Weiss R, Loth A, Leinung M, Balster S, Hirth D, et al. (2020) A new adhesive bone conduction hearing system as a treatment option for transient hearing loss after middle ear surgery. Eur Arch Otorhinolaryngol 277(3): 751-759.
- Dahm V, Auinger AB, Liepins R, Baumgartner WD, Riss D, et al. (2019) A Randomized Cross-over Trial Comparing a Pressure-free, Adhesive to a Conventional Bone Conduction Hearing Device. Otol Neurotol 40(5): 571-577.
-
Rami Abu Dakah, Dörte Fischer and Tino Just*. Prospective, Monocentric, Repeated-Measures Study of ADHEAR in Adults with Middle Ear Disease. On J Otolaryngol & Rhinol. 6(4): 2024. OJOR.MS.ID.000645.
-
Middle ear disease; Hearing; Audio processor; Hearing aids; Ear; Retrocochlear; ADHEAR system; Speech intelligibility; Ear canal
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






