 Research Article
Research Article
Mesenchymal Neoplasms with GLI1 Gene Alterations, A Novel Diagnostic Entity in the Head and Neck Region
Olivier Janjic1*, Claudio De Vito, MD, PhD2, Johannes Alexander Lobrinus, MD2, Minerva Becker, MD3 and Nicolas Dulguerov, MD1
1Department of Oto-Rhino-Laryngology-Head and Neck Surgery, Geneva University Hospitals, Geneva, Switzerland
2Department of Clinical Pathology, Geneva University Hospitals, Geneva, Switzerland
3Department of Imaging and Medical Information Sciences, Geneva University Hospitals, Geneva, Switzerland.
Olivier Janjic, Nicolas Dulguerov, Department of Oto- Rhino-Laryngology-Head and Neck Surgery, Geneva University Hospitals, Geneva, Switzerland.
Received Date:January 26, 2024; Published Date:February 08, 2024
Abstract
GLI1 gene alterations have recently been identified as a pathological phenomenon associated with a distinct novel entity of mesenchymal neoplasms. They have been reported to occur in any soft tissue of the body, with a specific affinity for the head and neck region. Herein, we report the case of a 39 year-old male patient with a ACTB::GLI1 fusion related mesenchymal tongue tumour along with a complete review of the literature on head and neck GLI1-altered mesenchymal tumours.
Introduction
GLI1 gene alterations have lately been identified as a pathological phenomenon associated with a particular type of soft tissue neoplasms. These tumours have a wide anatomic distribution and can develop in any part of the body, but a significant proportion are located in the head and neck region. Of these, a large majority (70-80%) are found in the tongue [1]. GLI1 is a member of the GLI gene family, responsible for encoding the GLI1 transcription factor, which is a significant downstream effector within the hedgehog cascade. A multitude of tumours, including glioblastoma multiforme, hepatocellular carcinoma, gastric cancer and rhabdomyosarcomas exhibit aberrant hedgehog signalling activation [2]. Reported GLI1 alterations consist in its amplification or its translocation with numerous partners, whose number has constantly been increasing according to the recently published works. GLI1-altered mesenchymal tumour is a new diagnostic entity of which all surgeons operating in the head and neck region, radiologists and pathologists should now be aware, especially when dealing with an atypical presentation of a head and neck mass. Until recently, this entity was unknown and these lesions, once resected and analysed, could easily be misdiagnosed or categorised as “unclassified tumours”. To date, the vast majority of cases described in the literature have been retrieved from past consultations and diagnosed retrospectively. Due to recent work on the subject, we were able to diagnose an ACTB- GLI1 fusion related tongue tumour in a 39-year-old male patient. Despite the significant attention paid to histopathological and molecular analysis in past research, there is currently limited clinical description available for this uncommon condition. Through a complete literature review, the aim of this article is to increase the awareness of this entity and to provide a detailed summary of the modes of presentation as well as the diagnostic and therapeutic issues surrounding these tumours occurring in the head and neck region.
Case Report
Clinical history
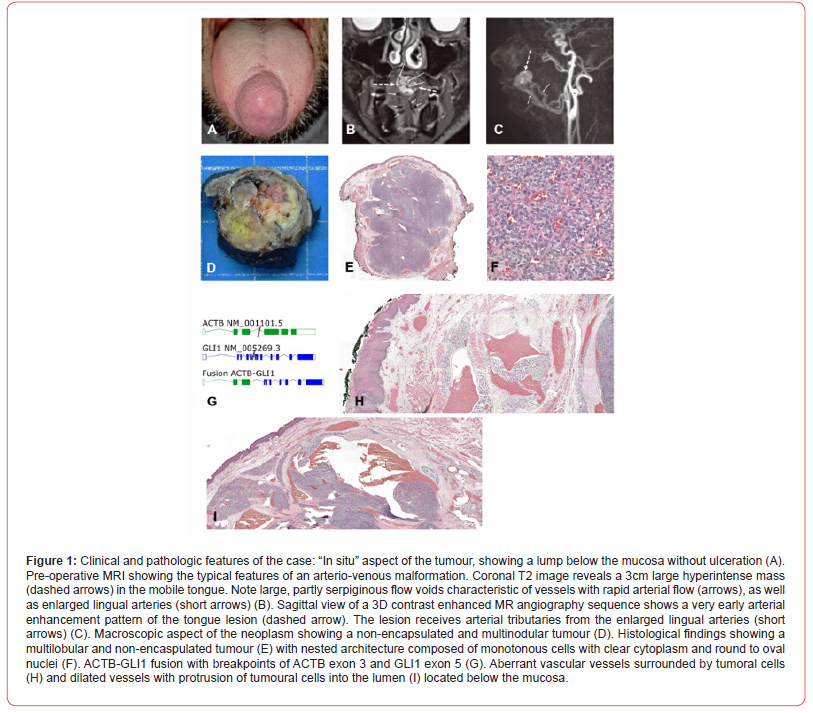
At the Department of Otolaryngology of the University Hospital of Geneva, we studied the case in 2020 of a healthy 39-year-old male patient who did not drink or smoke. He presented to the ENT clinic with a mass at the tip of the tongue that had been growing progressively since the age of 12. Intraoral examination revealed a voluminous tumour measuring approximately 1.5 x 2cm in horizontal dimension and approximately 1cm deep. It was soft, firm and covered by the tongue mucosa, which did not show any change (Figure 1A). The rest of the physical examination was normal: in particular, no lymphadenopathy was found. The patient stated that he had previously consulted general practitioners, who did not conduct any additional investigations due to the lack of symptoms. During our examination, the patient presented with a mild speech impairment and occasional tongue biting. He did not report any pain or weight loss. We conducted a head MRI (details below), which was initially consistent with a benign arterio-venous vascular malformation. No incisional biopsy was undertaken, and the case was consequently presented to the tumour board, who opted to solely treat via surgery. A median glossectomy was performed and the tumour was successfully removed in toto with negative, although near margins. A two-year follow-up, including multiple skull MRIs and thoraco-abdominal CT scans, revealed no clinical or radiological evidence of tumour recurrence.
Imaging
We performed an MRI with dynamic TWIST sequence after contrast injection (Dotarem). The examination showed a lingual mass, centred on the anterior third of the dorsum of the tongue, measuring 19x24x20mm (AP x TR x CC) and spreading the fibers of the intrinsic musculature of the tongue. It showed no infiltration of the extrinsic musculature and extended inferiorly to the genioglossus muscle.
Its signal was heterogeneous with an hypointense signal in T2/STIR and isosignal in T1. The T1 images showed a fleshy component, with slight diffusion restriction, and homogeneous and strong enhancement. The images exhibited multiple internal serpiginous structures demonstrating flow-voids, which confirmed the vascular origin (some with a caliber up to 2mm). No significant edema was found nearby the lesion. The dynamic TWIST sequences showed early contrast of the lesion in arterial time, with rapid partial venous washout and persistent enhancement of the fleshy component (Figure 1B & C).
Pathology
Partial glossectomy was performed, which revealed a 3cm non-encapsulated whitish soft multinodular tumour (Figure 1D). Histologically, the neoplasm showed a nested architecture with a richly vascular network composed of monomorphic tumoral cells with round to oval nuclei, small nucleoli and a clear cytoplasm (Figure 1E & 1F). Protrusions of tumour cells into the vascular lumina were noted. No mitosis or necrosis were observed. Immunohistochemistry demonstrated weak and focal S100 staining. SMA, pankeratin, STAT6 were negative. GLI1 alteration was confirmed using the Illumina TruSight RNA fusion panel (Illumina, San Diego, CA), that demonstrated ACTB-GLI1 fusion with breakpoints of ACTB exon 3 and GLI1 exon 5 (Figure 1G). No evidence of MDM2, CDK4 or GLI1 amplification using Oncoscan CNV assay (ThermoFisher scientific, Waltham, MA) was noted. Altogether, these findings supported the diagnosis of head and neck mesenchymal neoplasm with GLI1 alterations.
We observed at the periphery of the neoplasm, below the mucosa, aberrant vascular structures surrounded by neoplastic cells around the vascular wall.
Methods
Search
To conduct the literature review, a computerised literature search was performed through MEDLINE/PubMed, Embase and the Cochrane databases, applying the following terms: “GLI1” (MeSH term), “mesenchymal tumour” (MeSH term), “Human Glioma-Associated Oncogene Homolog 1” (free text). These terms were combined using “AND”. Additional articles and abstracts were identified by cross-referencing. Articles published in English language from implementation of above-cited databases until now were considered for the present review.
Eligibility criteria
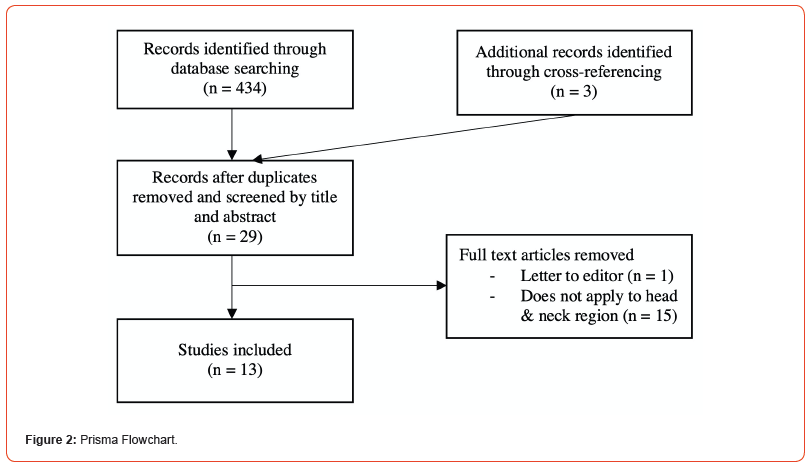
Papers concerning GLI1-altered soft tissue tumours were evaluated, and head and neck cases were analysed and extracted for the review. Only full-text articles were included. Letters to the editor, posters, and clinical images were excluded. This systematic review was conducted and reported following the PRISMA guidelines (Figure 2).
Data extraction
The following data were extracted: sex, age, site of tumour, clinical manifestation and its duration, radiological description, type of treatment and outcome. Tumour features were collected, such as its size and gross description, presence of necrosis, and GLI1 alteration type. Informed consent was obtained to publish the information/image of the case.
Results
A total of 13 articles on GLI1-altered soft tissue tumours were selected, of which 9 reported tumours located in the head and neck region. Including our case, 32 cases have been published so far, with a strong majority of males affected (25 males and 7 females). The median age was 34 years (IQR 34). Twenty-three tumours occurred in the tongue (of which 2 at the tongue base and one extending to the oropharynx), 3 in the neck, one in the submandibular gland, 2 at the mouth floor and one each in a palatine tonsil, on the soft palate and in the oropharynx (not otherwise specified). Out of the 21 cases in which treatment details were specified, tumours were primarily removed through surgery. In two cases, chemotherapy was attempted but had no significant effect, so surgery was carried out instead [3]. Adjuvant radiotherapy and chemotherapy were given to two patients. In one case, the decision was made due to local recurrence [4]. Follow-up has been carried out for 17 patients during a period that ranged between 2 to 120 months (mean 31 months). Three patients showed local recurrence with distant and/ or regional metastasis while one patient had local recurrence only [1, 5]. The rest showed no evidence of disease at their last follow-up consultation. Tumour size was described in 17 patients and ranged from 0.8 to 5.8cm (median 2.4cm, mean 2.87cm) [3-8]. Tumour necrosis was documented for every patient and has been found in 2 cases [1].
Clinical features such as radiological assessment and findings, gross description of the tumour and patient symptoms were scarcely reported. Essentially, the patients described a growing painless mass causing no other discomfort except the ones created by the mechanical expansion in itself (speech impairment, dysphonia, dysphagia) [4, 5, 7]. Our case is an exception concerning the tumour growing time (27 years) as the other reported cases varied from 1 to 6 months [4, 5, 7]. Macroscopically, the tumours appear capable of harbouring diverse presentations. The texture varied from fleshy and indurated to spongy, with the most commonly reported colours being brown, white, and grey [4-7, our study]. Though these tumours are generally considered indolent, some presented with ulceration. Radiological findings were only reported in two patients and findings were rather non-specific, describing heterogenous tumours on injected CT-scan [5, 7]. Clinicopathological results are partly summarized in Table 1.
Table 1:Clinicopathologic Characteristics of 32 Head and Neck GLI1-altered tumours.
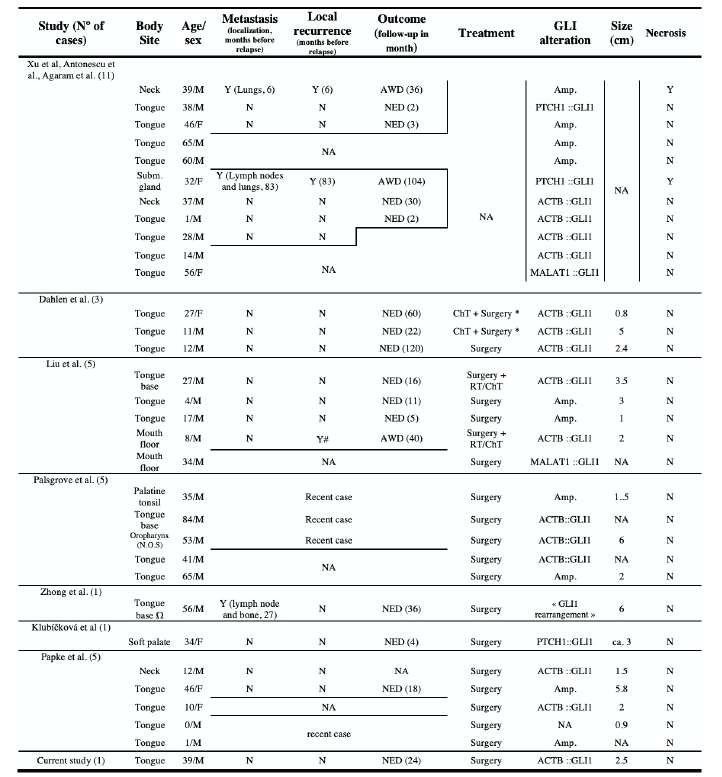
Y = Yes, N = No, NA = not available, NED = No Evidence of Disease, AWD = Alive With Disease, RT = Radiotherapy, ChT = Chemotherapy, Amp. = Amplification, # : Local recurrence occurred twice, once at 27 and a second time at 40 months, * Surgery was performed after an unsuccessful attempt with ChT, Ω tumour extending to the right oral tongue and lateral pharyngeal wall
Discussion
Primary soft tissue tumours harbouring alterations of the GLI1 gene have been increasingly reported in recent series. In addition to the head and neck region, they can occur in various locations throughout the body, including the kidneys, stomach, duodenum, small intestines, uterine cervix, ovaries, bones, skin and muscles [1, 4, 6, 8-16]. In 2004, Dahlen, et al. [3] described tumours with distinctive pericytic features harbouring GLI fusion with ACTB gene, called “pericytoma with t(7;12)” [3]. Since then, other authors reported tumours with similar histological and clinical features associated with new mechanisms of GLI alteration such as GLI1 amplification (often combined with co-amplification of DDIT3, CDK4, and MDM2) or fusion with new partners. As progress is made in this field, the list of fusion partners is getting longer and currently comprises the following genes: MALAT1, PTCH1, DERA, APOD, FOXO4, HNRNPA, TXNIP, NEAT1 and ABCA1 [6, 8, 17-21].
Various terms have been proposed so far to better nominate this emerging entity, such as “GLI1-altered mesenchymal tumour “ by Liu, et al. [4] “GLI1-altered epithelioid soft tissue tumour” by Zhong, et al. [5] or “sarcoma of unknown lineage with GLI1alteration” by Xu, et al. [1, 4, 5] Histologically, those neoplasms share specific features such as monomorphic, bland cells, prominent capillary networks and proliferation of tumour cells around vessels in soft tissue surrounding the tumour [5]. However, regarding immunohistochemical analysis, GLI-1 altered tumours in different anatomical locations have not demonstrated a uniform profile yet and have proven to be unreliable for diagnosis purposes [5, 15, 22]. Papke, et al. recently reported a series of 20 new cases harbouring GLI1 altered genes that they named “distinctive nested glomoid neoplasms” and classified as a subset within the larger “GLI1 altered mesenchymal tumour” group [6]. In their view, those nested glomoid neoplasms might represent a benign entity that resembles glomus tumours or well-differentiated neuroendocrine tumours. This subgroup could co-exist on a biological continuum with “pericytoma with t(7;12)” as they share certain genetical, clinical and histological characteristics but differ in others ; nested glomoid tumour would rather display nested architecture and round-toovoid cytomorphology while “pericytoma with t(7;12)” would show prominent microcystic spaces and pseudopapillary architecture [6, 23]. As the aggressiveness of GLI1 altered mesenchymal tumours appears to be quite heterogeneous, with some tumours growing slowly for years and others causing distant metastases rapidly, the authors have attempted to identify clinical or pathological features that may be of prognostic value.
Xu, et al. [1] suggested that tumour necrosis and an anatomical site outside the oral tongue could be linked to aggressive behaviour. Tumour necrosis was well documented in every reviewed article but was found in only two tumours; those tumours gave rise to metastases [1]. However, two other tumours without necrosis also showed aggressive behaviours (local recurrence and distant metastasis) [4, 5]. Regarding prognostic predictions based on anatomical location, our data potentially supports the proposition of Xu et al. as only tumours situated outside of the oral tongue exhibit aggressive behaviour [1, 4, 5]. Yet, among cases with reported follow-up, three tumours located outside the oral tongue did not demonstrate any sign of local recurrence or metastasis within a mean follow-up period of 20.3 months. In addition, ten tumours were situated in the oral tongue and they did not show any relapse after a mean follow-up of 26.5 months. Altogether, subject to clinical gaps, this may suggest that necrosis and an anatomical site outside the oral tongue are not essential factors for aggressive behaviour but may promote it. This might also imply that a location in the oral tongue could be linked to a benign evolution.
In the reviewed articles, very few clinical descriptions have been provided, especially concerning diagnostic procedure and radiological findings. Although mesenchymal tumours with altered GLI1 form a rare pathological entity, a consistent description of radiological assessment may provide diagnostic and/or prognostic value when reporting such diseases in articles. The role of radiology in diagnostic procedures cannot be disregarded since cases are often ultimately diagnosed by radiologists or based on their recommendations. While diagnostic imaging alone may not be sufficient for the diagnosis of most rare diseases, such assessments serve as critical inputs in the diagnostic procedure. In our case, MRI findings showed multiple internal serpiginous structures demonstrating flow-voids, which made us primarily suspect a vascular origin for the tumour. The identification of aberrant vascular vessels might serve as a radiological feature that could potentially facilitate the challenging process of diagnosing a rare disease such as GLI altered mesenchymal tumour.
As papers on the topic were based on data retrieved from past consultations, the available clinical information was generally incomplete and sometimes entirely lacked follow-up data. In addition, some cases have only recently been diagnosed, and there has not been enough time for relevant outcomes to emerge. With recent interest in this field, it is our hope that extensive future studies and larger series are underway. Such analyses will be crucial in defining the clinicopathological range of this novel entity, especially since certain subgroups may exhibit malignant behaviour.
In conclusion, we successfully treated a symptomatic and long lasting ACTB-GLI1 fusion mesenchymal tumour of the tongue. Our radiological work-up initially indicated a low-flow arterio-venous vascular malformation. Histologically the neoplasm displayed a nested architecture with a rich vascular network composed of monomorphic tumour cells with round to oval nuclei. The described histological features are consistent with the “ distinctive nested glomoid neoplasms” group. Furthermore, the benign status of the tumour reinforces this resemblance. We suggest a followup period of at least 2 years with imaging followed by a clinical follow up of 3 years. The longest reported relapse onset was 83 months, hence the recommended follow-up duration [1]. Subject to lack of strong evidence, features suggestive of malignancy, such as location outside the tongue, presence of necrosis, or a histological description similar to “pericytoma with T(7;12)”, may warrant further and more extensive follow-up.
Data availability statement
All data generated or analysed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Author responsibilities
OJ and ND wrote the main manuscript text. OJ prepared figure 2 and table 1. CD, JL and MB prepared figure 1. All authors reviewed the manuscript.
References
- Xu B (2020) Head and Neck Mesenchymal Neoplasms With GLI1 Gene Alterations : A Pathologic Entity With Distinct Histologic Features and Potential for Distant Metastasis. Am J Surg Pathol 44(6): 729‑
- Chetty R (2020) Gene of the month: GLI-1. J Clin Pathol 73(4): 228‑
- Dahlen A (2004) Activation of the GLI Oncogene through Fusion with the β-Actin Gene (ACTB) in a Group of Distinctive Pericytic Neoplasms. Am J Pathol 164(5): 1645‑
- Liu J, Mao R, Lao IW, Yu L, Bai Q, et al. (2022) GLI1-altered mesenchymal tumor : A clinicopathological and molecular analysis of ten additional cases of an emerging entity. Virchows Arch 480(5): 1087‑
- Zhong H, Xu C, Chen X, Guo X, Yang S (2022) GLI1-altered epithelioid soft tissue tumor : A newly described entity with a predilection for the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol 134(1): e14‑
- Papke DJ, Dickson BC, Oliveira AM, Sholl LM, Fletcher CDM (2023) Distinctive Nested Glomoid Neoplasm:Clinicopathologic Analysis of 20 Cases of a Mesenchymal Neoplasm With Frequent GLI1 Alterations and Indolent Behavior. Am J Surg Pathol 47(1): 12‑
- Klubíčková N (2022) Epithelioid Soft Tissue Neoplasm of the Soft Palate with a PTCH1-GLI1 Fusion : A Case Report and Review of the Literature. Head Neck Pathol 16(2): 621‑
- Nitta Y, Takeda M, Fujii T, Itami H, Tsukamoto S, et al. (2021) A case of pericytic neoplasm in the shoulder with a novel DERA–GLI1 gene fusion. Histopathology 78(3): 466‑
- Castro E, Cortes Santiago N, Ferguson LMS, Rao PH, Venkatramani R, et al. (2016) Translocation t(7;12) as the sole chromosomal abnormality resulting in ACTB-GLI1 fusion in pediatric gastric pericytoma. Hum Pathol 53: 137‑
- Kerr DA(2019) Pericytoma With t(7;12) and ACTB-GLI1 Fusion : Reevaluation of an Unusual Entity and its Relationship to the Spectrum of GLI1 Fusion-related Neoplasms. Am J Surg Pathol 43(12): 1682‑
- Koh NWC, Seow WY, Lee YT, Lam JCM, Lian DWQ (2019) Pericytoma With t(7;12) : The First Ovarian Case Reported and a Review of the Literature. Int J Gynecol Pathol 38(5): 479‑
- Prall OWJ (2020) A Malignant Neoplasm From the Jejunum With a MALAT1-GLI1 Fusion and 26-Year Survival History. Int J Surg Pathol 28(5): 553‑
- Zeng Y, Yao H, Jiang X, Tang X, Wang X (2023) GLI1-altered Mesenchymal Tumor Involving the Duodenum : Case Report and Literature Review. Int J Surg Pathol 31(8): 1538‑
- Argani P (2022) GLI1 Gene Alterations in Neoplasms of the Genitourinary and Gynecologic Tract. Am J Surg Pathol 46(5): 677‑687.
- Bridge JA (2012) Pericytoma with t(7;12) and ACTB-GLI1 fusion arising in bone. Hum Pathol 43(9): 1524‑
- Rollins BT, Cassarino DS, Lindberg M (2022) Primary cutaneous epithelioid mesenchymal neoplasm with ACTB‐GLI1 fusion : A case report. J Cutan Pathol 49(3): 284‑
- Agaram N (2019) GLI1-amplifications expand the spectrum of soft tissue neoplasms defined by GLI1 gene fusions. Modern Pathology 32(11): 1617‑
- Antonescu CR, Agaram NP, Sung YS, Zhang L, Swanson D, et al. (2018) A Distinct Malignant Epithelioid Neoplasm With GLI1 Gene Rearrangements, Frequent S100 Protein Expression, and Metastatic Potential : Expanding the Spectrum of Pathologic Entities With ACTB/MALAT1/PTCH1-GLI1 Fusions. Am J Surg Pathol 42(4): 553‑
- Pettus JR (2021) Primary myxoid and epithelioid mesenchymal tumor of the kidney with a novel GLI1‐FOXO4 Genes Chromosomes Cancer 60(2): 116‑122.
- Lopez Nunez O, Surrey LF, Alaggio R, Herradura A, McGough RL, et al. (2021) Novel APOD – GLI1 rearrangement in a sarcoma of unknown lineage. Histopathology 78(2): 338‑
- Agaimy A (2020) What is new in epithelioid soft tissue tumors?. Virchows Archiv 476(1): 81-96.
- Palsgrove DN (2022) GLI1-Altered Soft Tissue Tumors of the Head and Neck : Frequent Oropharyngeal Involvement, p16 Immunoreactivity, and Detectable Alterations by DDIT3 Break Apart FISH. Head and Neck Pathol 16(4): 1146‑
- Kerr DA (2019) Pericytoma With t(7;12) and ACTB-GLI1 Fusion : Reevaluation of an Unusual Entity and its Relationship to the Spectrum of GLI1 Fusion-related Neoplasms. Am J Surg Pathol 43(12): 1682‑
-
Olivier Janjic, Claudio De Vito, MD, PhD, Johannes Alexander Lobrinus, MD, Minerva Becker, MD and Nicolas Dulguerov, MD. Mesenchymal Neoplasms with GLI1 Gene Alterations, A Novel Diagnostic Entity in the Head and Neck Region. On J Otolaryngol & Rhinol. 6(4): 2024. OJOR.MS.ID.000644.
-
Head and neck, Mesenchymal neoplasms, Hepatocellular carcinoma, ENT clinic, Oral tongue, Tumours, Immunohistochemical analysis, Mucosa, Otolaryngology.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






