 Research Article
Research Article
Spatial Distribution and Ecological Niche Modelling of Some Potential Vectors Involved in the Transmission of Buruli Ulcer in Benin (West Africa)
Akoton Tété Pérugine*, Koura Kourouma and Ganglo Cossi Jean
Laboratory of Forest Sciences, University of Abomey-Calavi, Benin, West Africa
Akoton Tété Pérugine, University of Abomey-Calavi, Faculty of Agronomic Sciences, Master’s Program in Biodiversity informatics, Laboratory of Forest Sciences, Abomey-calavi Atlantique, Benin, West Africa.
Received Date: May 16, 2023; Published Date: June 19, 2023
Abstract
Buruli Ulcer (BU) is an infectious disease caused by a pathogen, Mycobacterium ulcerans. This mycobacterium is responsible for severe necrosis of subcutaneous tissue leading to chronic ulcers and wounds. However, the distribution of potential reservoirs of this mycobacterium, which is necessary for an effective dynamics control, remains poorly known in Benin. This study aims to model the spread of BU infection risk in Benin. The methodological approach focused on data collection, processing and analysis. Indeed, two data sources were used in the MaxEnt (Maximum Entropy) software to model the spatial distribution of some families of Mycobacterium ulcerans vectors. These are present and future bioclimatic data (scenarios rcp 45 and rcp 85) downloaded from http://webfiles.york.ac.uk/KITE/AfriClim/ and occurrences (234) of the disease from fieldwork and literature. Potential vectors inventoried in the field are aquatic bugs of the family Belostomatidae and Naucoridae and worms whose taxa were not specified. The areas at risk of this disease in the present as well as in the future, globally include the South to the Center Benin and parts of the North-West and North-East of the country. The various results obtained are valuable for prevention and better management of patients. To this end, it will be necessary to focus attention and strengthen control interventions in areas at present and future risk of the disease.
Keywords:Buruli ulcer; Risk map; Modeling; Mycobacterium ulcerans; Benin
Abbreviations: BU: Buruli Ulcer; MU: Mycobacterium ulcerans; ROC: Receiver Operating Characteristic; AUC: Area Under Curve (AUC); GBIF: Global Biodiversity Information and Facility
Introduction
Climate change is recognized as the greatest threat to the health system [1]. The main risks are related to several areas including changes in the distribution of pathogens and vectors [2]. The manifestations of climate change have effects on living beings in general but, also participate in the multiplication of some disease vectors in the world and in Africa particularly. Thus, health and climate are linked and are at the heart of the concerns of our human societies. As climate is one of the components of the ecological niche of species, a linear conception of the impacts of climate change on health has long prevailed [3]. Health risks are indicative of changes related to interactions between humans, biodiversity and their environment. Mapping risks is therefore an essential step in setting up procedures for managing foreseeable health crises with the aim of reducing their frequency and impact on the population [4]. However, to facilitate disease control, it is often important to understand the spatial distribution of pathogens and their vectors.
Buruli ulcer (BU) is a chronic debilitating infection caused by Mycobacterium ulcerans. Indeed, the lack of data on the mode of transmission of the disease as well as the existence of potential vectors severely hampers research, effective management and control efforts. Benin has a hyper-endemic profile for BU and ranks third among the most affected countries in BU history after Côte d’Ivoire and Ghana [5]. In Benin, recent statistical data still show a predominance of severe cases of the disease, a rate of 59 % of all patients screened in 2018 [6].
Despite the progress made in recent years, especially on understanding the risk factors of BU and its distribution [7-9] answers to the question of the effects of climate change on the habitats of the pathogen, its vectors, and predictions in terms of future projection of areas of high endemism are not yet enough well known. Ecological niche models are widely used to describe the spatial distribution of macro-organisms, such as birds or mammals, and in the last decade their use has increased considerably in the study of diseases, pathogens and their vectors [10]. Ecological niche modeling is often used to guide conservation decisions [11] to describe the potential range of species [12]. It is increasingly used to describe the spatial distribution and ecological niches of vectors of public health concern [13].
Recent techniques from Geographic Information Systems, remote sensing, and statistical techniques are reliable for considering more satisfactory distribution models. These different ecological niche distribution models of plant species, animals, bacteria, etc., provide a better explanation of the ecology of potential vectors on the one hand, and on the other hand, allow the development of the risk map for disease spread. Among the modelling methods, we have the Maximum Entropy (MaxEnt) [14, 15]. Which is an algorithm for developing spatial models including the geographical prediction of the distribution areas of living organisms. This algorithm uses only presence data, which is more frequently used in ecology compared to presence-absence data; and using many interacting variables [16].
The present study was initiated to contribute to the knowledge of environmental factors and to have a database on spatial distribution on the one hand and to have information on the ecological niche of potential vectors incriminated in the transmission of Buruli ulcer in Benin using MaxEnt algorithm on the other hand. It is also important in that it allows us to generate an effective tool for predicting the areas that are likely to be colonized by the Mycobacterium ulcerans responsible for Buruli ulcer.
Study area
The work within the scope of this study started from August 23, 2019 to July 1, 2020.
The Republic of Benin is the setting considered in this study. Located in West Africa, Benin covers an area of 114763 km2 and for a population of 10,008,749 inhabitants in 2013 [17]. The republic of Benin is located in the tropical zone between the equator and the tropic of cancer, between latitudes 6°30’ and 12° 30’ North and longitudes 1° and 3°40’ East. The climate is tropical, hot and humid on the whole, with seasonal and geographical nuances imposed by the country’s extension in latitude, relief and alternating seasons.
Of the climatic zones, the semi-arid Sudanian zone in the north is characterized by annual rainfall ranging from 900 mm to 1100 mm with a high rainfall deficit. The average temperature in this zone is 27.5°C. The Sudano-Guinean zone in the center, between latitudes 7° and 10° north, is characterized by very marked temperature fluctuations and an average annual rainfall of 1200 mm. The average annual temperature is 27°C. Finally, the Guinean zone to the south, between 6°30 and 7° North, has rainfall ranging from 900 mm to 1500 mm per year for an average annual temperature of 26.5°C. The risk map for the spread of Buruli ulcer was produced for Benin. Data concerning the vectors of BU (water bugs of the Belostimatideae and Naucorideae families) were collected in some departments (Table 1).
Table 1:Sampling commune.
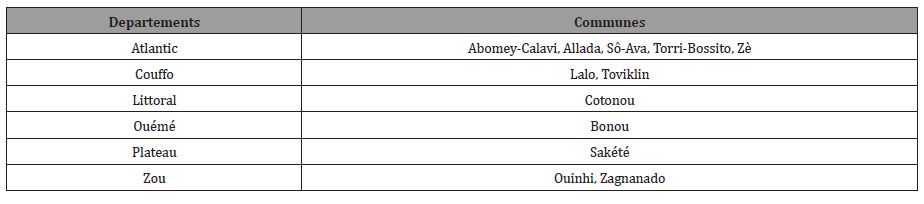
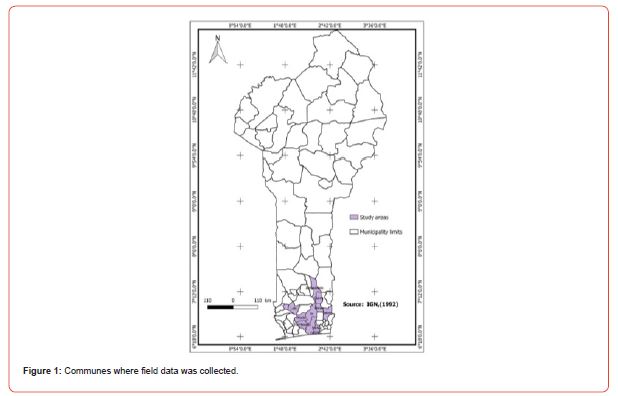
Benin includes (12) twelve departments of which eight (08) are endemic for BU (Atlantic, Littoral, Mono, Couffo, Zou, Collines, Ouémé and Plateau) all located in the southern and central part of the country. Indeed, most cases of Buruli ulcer were observed near rivers including the Couffo River, the Mono River, and the Ouémé River (Figure 1) [18]. Note that the southern part of Benin is endowed with an important hydrographic resource including lakes, rivers, swamps, ponds, lowlands, etc. (Figures 1, 2)
Material and Methods
Material
The work within the scope of this study started from August 23, 2019 to July 1, 2020.
The Global Positioning System (GPS receiver) was used to take the geographical coordinates of the households of people who were ill or cured of BU and also of potential reservoirs (open-air water points) close to the households. These different reservoirs are considered to be the locations of potential UB vectors. In addition to the GPS receiver, illustration boards of potential disease vectors were developed to facilitate communication with the target groups. A field guide was used to collect the data. QGIS 2.18.4 software was used for processing and various cartographic analyses.
The snowball method was adopted to identify the target persons and also a random sampling based on the data available at the community relays. A total of 100 people were surveyed, of which 74 were cured, 21 had BU and 5 had relapsed. The habitat of the potential vectors was a water environment (small ponds, swamps, streams/rivers and lakes). These different environments were identified based on the respondents whose geographic coordinates were taken. The distance between water points and households was assessed to evaluate correlations between households and water bodies.
Modeling the spatial distribution and ecological niche of potential BU vector
Points of occurrence
Two data sources were used to model the spatial distribution and ecological niche of potential BU vectors in this study. Two hundred and thirty-four (234) occurrence points were used to calibrate the model with MaxEnt [14]. These were data collected in the field and data from the literature. Those data are now published on GBIF site (https://doi.org/10.15468/nh3jbw).
Environmental variables
Present and future bioclimatic data were downloaded on October 03, 2019 from Africlim (https://webfiles.york.ac.uk/KITE/ AfriClim/GeoTIFF_30s/; Platts et al., 2015); under the rcp 4.5 and rcp 8.5 scenarios, horizon 2055 at 30-second resolution. Elevation data were downloaded from https://globalmaps.github.org. A mask was generated with MaxEnt to minimize bias through a polygon shapefile that covers the study area. Next, model calibration was performed with the environmental variables. An alignment of the layers was performed to bring them to the same spatial resolution. After this step, conversion to ASCII was done using Quantum QGIS software. Selection of appropriate environmental variables was done using the Receiver Operating Characteristic (ROC) curve, the Area Under Curve (AUC) curve14, the table of percentage contributions of variables, and the Jackknife test.
Results
Risk factors for the disease
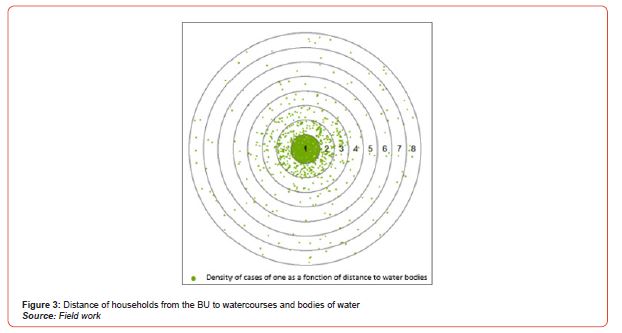
The distance of households from potential reservoirs is a factor in the spread of the disease. (Figure 3) shows the distance in kilometers (km) from households in the BU to water bodies. The figures represent the distance in km that separates households from reservoirs. From the analysis of the figure, it is notable that as one moves closer to water bodies, the number of households affected by BU increases. Thus, wetlands are favorable areas for potential vectors. (Figure 3)
cological niche model of potential BU vectors
Distribution of occurrences of Buruli ulcer
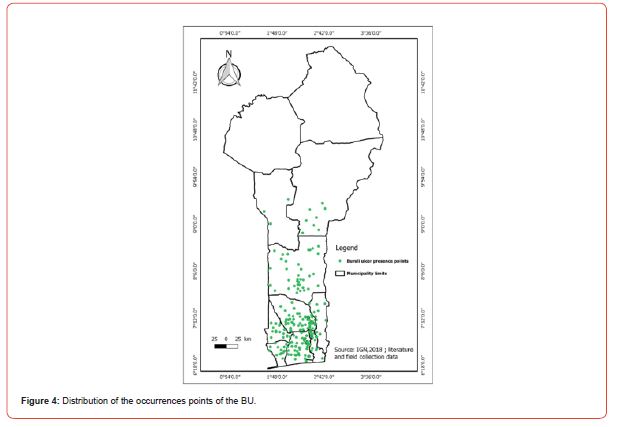
Modeling and model validation
In addition to the bioclimatic variables, the elevation, which is related to the topography, was chosen. These variables were chosen given the ecology of the vectors suspected in the spread of BU. Boostrap was used for the data selection mode with the MaxEnt program to evaluate the relevance of the variables. The model was tested with 25% of the occurrence data. Regarding the future distribution of the disease, the two scenarios used are RCP 4.5 which is the realistic scenario and RCP 8.5 which is the most pessimistic scenario by 2055.
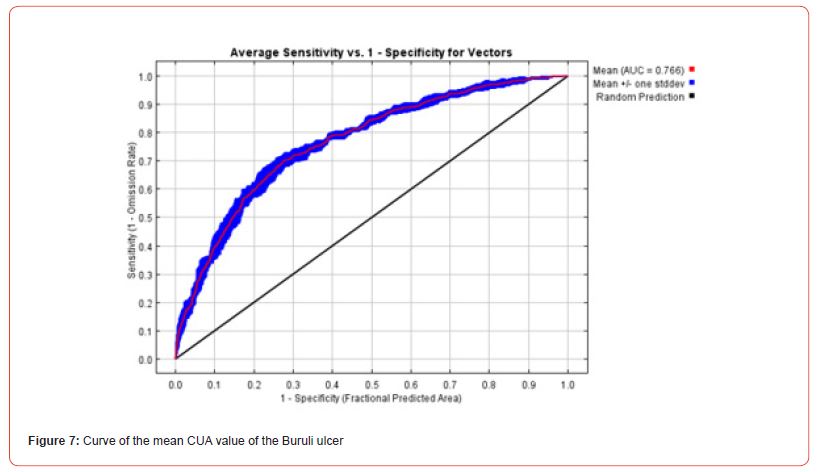
Model performance was evaluated using several methods. First, it was determined by assigning a subset of the occurrence data (75%) to the model calibration and using the remainder to test it (25%). Because performance can vary depending on the dataset selected for model calibration and testing, the MaxEnt algorithm was run 5 times and the average AUC value was equal to 0.766. This indicated that the model performed better than a random one. The “Partial ROC” test was applied for the validation of the model. The results of the Partial ROC test showed that after 500 simulations, the average value of the AUC ratio for an omission rate of 0.05 is 1.74 and that of AUC is 0.87. The True Skill Statistic (TSS) test was used to assess decision thresholds. The TSS values corresponding to the thresholds of ecological minimum conditions of species presence (0.116) and the maximum sensitivity and specificity (0.412) were 0.012 and 0.41, respectively. These TSS values confirmed that the model performed better than the random model.
Ecological niche model of potential BU vectors
Variable selection
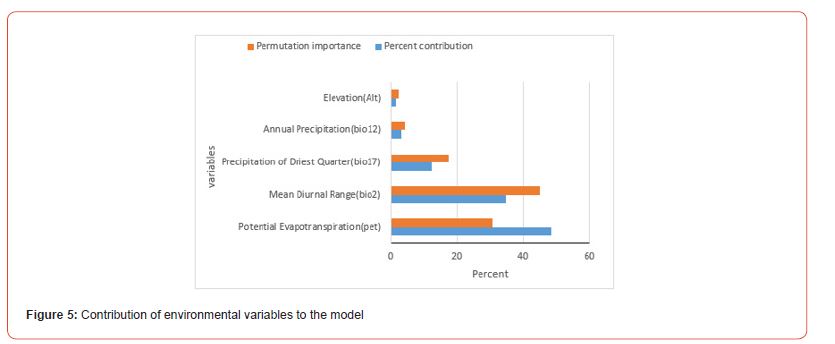
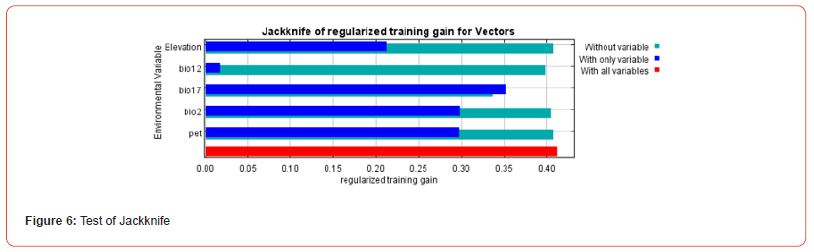
The distribution of potential BU vectors is controlled by bioclimatic and topographic variables. Aquatic bugs (families Belomastideae and Naucorideae) are insects that belong to the order Aquatic Hemiptera that usually live in swamps, ponds, or small, slow-moving streams. They thrive in areas where the average annual temperature ranges from 15 to 22°C, with moderate annual precipitation (i.e. 350 to 1000 mm/year). Selection of appropriate environmental variables was made using the Receiver Operating Characteristic (ROC) curve, the Area Under Curve (AUC) curve, the table of percentage contributions of variables, and the Jackknife maps to assess the contribution of variables (Figures 5-7).
Risk maps for the spread of Buruli ulcer
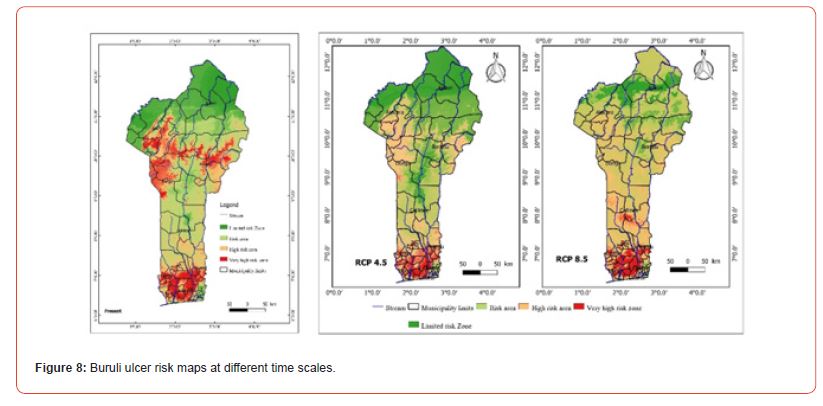
From the analysis of Figure 8, it appears that the dark red areas illustrate the areas of high Buruli Ulcer prevalence in Benin. From the analysis of this figure, it can be deduced that southern Benin is at risk in the present and the future. Predictions for the year 2055 (RCP8.5) showed that the departments of central Benin and Borgou (East Borgou) will also be affected by the disease. The departments of Donga, Atacora, and Borgou are overall areas at very high risk of Buruli ulcer in the present, but in the future this part of the country will no longer be part of the risk areas due to climatic parameters.
Discussion
Ecology of potential vectors and main environmental factors governing their ecological niches
The studies have identified potential habitats for the vectors of Buruli ulcer (BU) as wetland areas such as low-flow rivers, floodplains, ponds, and marshes. The environmental factors influencing the ecological niche of these vectors include bioclimatic variables such as mean diurnal temperature range, precipitation of the driest month, annual precipitation, potential evapotranspiration (PET), and altitude [19]. Several studies have shown that precipitation of the driest month, annual precipitation, PET, and altitude are consistent with the characteristics of potential habitats for BU vectors. These findings are in line with previous research that has also emphasized the importance of geoclimatic and physical factors in the presence of the bacteria and its vectors [19]. For example, a study conducted in Zoukougbeu, Côte d’Ivoire, demonstrated that the presence of the pathogen and its vectors was related to physical characteristics such as topography, hydrographic network, vegetation, and precipitation [20]. Another study focusing on modeling the spatial distribution of aquatic insects potentially involved in the transmission of Mycobacterium ulcerans in Africa also considered factors such as vegetation, topography, bioclimatic variables (including annual precipitation, maximum temperature, mean temperature, minimum temperature, mean temperature of the coldest quarter, mean temperature of the warmest quarter, precipitation of the driest quarter, precipitation of the wettest quarter, PET, aridity index, altitude, slope, stream accumulation, topographic humidity index, distance to watercourses, distance to water bodies, land surface temperature, enhanced vegetation index, and main land cover types such as forests, agriculture, and bush-grass) [17]. In summary, the research supports the influence of environmental factors such as geoclimatic, physical, and bioclimatic conditions on the presence of Buruli ulcer vectors and the bacteria itself.
Impact of climate and global changes on disease distribution and uncertainty of results
The study conducted risk mapping for the spread of Buruli ulcer (BU) and identified areas at risk both currently and in the future. The high-risk areas for BU include the southern and central regions of Benin, as well as parts of the northwestern (Atacora department) and northeastern (Borgou department) regions. Presently, the departments of Mono, Atlantic, Plateau, Couffo, and parts of Ouémé are at very high risk for BU. In particular, the communes of Lalo, Aplahoué, Djakotomey, Dogbo, Klouékanmè, and Toviklin in the department of Couffo remain at risk for the disease in the present and future (up to 2055). However, these findings contradict the results of another study [6], which projected an 80% reduction in BU risk areas by 2050. The contradiction can be attributed to differences in the methodological approach and the geographical coverage of the studies. The authors of study 6 relied on data from the National Program for the Control of Neglected Tropical Diseases and identified endemic villages as occurrence points of disease vectors. In contrast, the present study considered occurrence points at the household level, including cases of sick individuals, cured individuals, and relapse cases, which provides more realistic and precise data. Additionally, study [6] focused only on the commune of Lalo, limiting the scope of their results and failing to account for the diversity of environmental conditions in other localities where BU occurs in Benin. This discrepancy may explain the differences between the two studies’ findings.
Strategies to combat the spread of the disease in the context of climate and global changes
Strategies are being developed to combat the spread of the disease in the context of climate and global changes. These strategies include awareness campaigns, early detection of the disease, improving water quality, constructing latrines, providing materials such as boots, assisting disadvantaged populations, caring for the sick, and integrating care for Buruli ulcer patients into the primary healthcare system. Local-level strategies include handwashing with soap, wearing protective clothing and boots. These various strategies have also been developed in the National Master Plan for Integrated Control of Neglected Tropical Diseases [21]. This master plan places greater emphasis on measures such as early detection of disease cases, patient management, and hygiene promotion [18].
Other studies have also suggested measures to limit contamination and disease spread. For example, daily habits such as wearing long clothing, systematic use of mosquito nets, or the use of repellents can be effective [22-25].
According to our results, the areas at highest risk for Buruli ulcer, currently and in the future, require the implementation of these strategies and the reinforcement of control measures. These areas generally include the departments in the southern part of Benin. Control measures should also be concentrated in the departments where the disease will spread. At the individual level, measures should be taken to avoid bathing in stagnant water, maintain a clean environment, regularly spray the environment to control vectors, and use clean water and latrines.
Conclusion
BU is a disease that affects populations and is favored by the proximity of rivers and slow-flowing water bodies, the use of unprotected water sources for domestic activities, inadequate disinfection of wounds. The areas at risk of Buruli ulcer, both at present and in the future, include the south to Center Benin and parts of the North West of the country (Atacora department) and the North East (Borgou department). This study generated risk maps for the spread of the disease both at present and in the future. These results can be used to support the authorities in charge of health for a more effective fight against this tropical disease.
Acknowledgement
The authors of the manuscript sincerely thank JRS Biodiversity Foundation for its financial support to the master and Ph. D program of biodiversity informatics in Africa
Funding: JRS Biodiversity Foundation
Contribution: GANGLO Jean Cossi et KOURA Kourouma have all contributed to
• The conception or design of the work; or the acquisition, analysis, or interpretation of data for the work;
• Drafting the work or revising it critically for important intellectual content;
• Final approval of the version to be published;
• Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
The authors declare that no conflict of interest.
Informed Consent
The manuscript does not contain any individual person’s data in any form.
References
-
Akoton Tété Pérugine*, Koura Kourouma and Ganglo Cossi Jean. Spatial Distribution and Ecological Niche Modelling of Some Potential Vectors Involved in the Transmission of Buruli Ulcer in Benin (West Africa). Online J Ecol Environ Sci. 1(1): 2023. OJEES.MS.ID.000505.
-
Mycobacterium ulcerans, Distribution of pathogens, Buruli ulcer, Climate change, Global positioning system, Potential reservoirs, Bioclimatic variables, Aquatic hemiptera, Topography, Stream accumulation.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






