 Case Report
Case Report
First Reported Case of Human Infection with Chromobacterium Violaceum in Gabon: Antibiotic Susceptibility Patterns and Treatment Outcome
Romeo Wenceslas Lendamba1*, Pierre Philippe Mbehang Nguema2, Mesmin Yves Moussounda3, Gaël NSI4, Eveline Avoune Epse Ella Missang5, Dearie Glory Okwu1, Claude Bernard Ako’o Mve1, Jacques François Mavoungou2 and Ghyslain Mombo-Ngoma1,6
*1Centre de Recherches Médicales de Lambaréné (Cermel), Lambaréné, Gabon
2Research Institute for Tropical Ecology (IRET), CENAREST, Libreville, Gabon
3Hôpital d’Instruction des Armées Omar Bongo Ondimba (HIAOBO), Libreville, Gabon
4University of Sciences and Technology of Masuku (USTM), Franceville, Gabon
5Centre Hospitalier Régional Amissa Bongo, Franceville, Gabon
6Implementation Research Department, Bernhard Nocht Institute for Tropical Medicine (BNITM) & I. Dep of Medicine, University Medical Center Hamburg- Eppendorf (UKE), Bernhard-Nocht-Straße, Hamburg, Germany
Romeo Wenceslas Lendamba, Centre de Recherches Médicales de Lambaréné (Cermel), Lambaréné, Gabon.
Received Date: July 11, 2023; Published Date: July 31, 2023
Abstract
Background:Mostly found in soil and still water in the subtropics and tropics, Chromobacterium violaceum is a Gram-negative bacillus that rarely infects humans. Nevertheless, when Chromobacterium violaceum infections do occur, they result in great distress because they are unrecognized and poorly addressed. The aim here is to describe what appears to be the first human case of Chromobacterium violaceum infection in Gabon.
Case presentation: A 12-month-old female was admitted to the paediatric ward of the Centre Regional Hospital Amissa Bongo of Franceville in Gabon with a fever that had been lingering for a week. The child was first put on antimalarial treatment for 3 days (following a positive thick blood smear for Plasmodium falciparum) combined with a 10-day prophylactic course of antibiotics (association of 500mg Ceftriaxone and Tobramycine).
Subsequently, a post-treatment cytobacteriological examination of urine (CBEU) was performed: a urine dipstick (UD) was carried out and then the urine was plated on Cystine-lactose-electrolyte-deficient (CLED) agar medium and incubated for 24 hours at 37°C.
Isolated bacterial colonies were identified with the Vitek 2 system (bioMerieux, France). Antibiotic susceptibility tests were run based on the Kirby-Bauer method (interpretation based on the breaking points given by the CLSI for Escherichia coli).
The UD revealed leukocyturia (30.103μL-1) and greyish non-pigmented bacterial colonies (positive for catalase and oxidase) found on CLED (DGU=104UFC.mL-1) were identified as Chromobacterium violaceum.
Antibiotic susceptibility tests exhibited resistance to beta-lactams (Ticarcillin, Ertapenem, Cefotaxime, Ceftazidime and Ceftriaxone), aminoglycosides (Gentamicin and Tobramycin), urinary quinolones (Nalidixic acid) and sulphonamides (Trimethoprim/Sulfamethoxazole).
Patient outcome: The infectious syndrome disappeared after the probabilistic treatment was adjusted post-CBEU and switched to Cefaclor 125 mg/5ml at a dosage of 25 mg/kg/day.
Conclusion: Chromobacterium violaceum seems to be a severe emerging human pathogen that requires prompt, adequate and lab results antibiotic-based treatment to prevent fatal outcomes.
Keywords:
Chromobacterium violaceum
; Urinary tract infection; Antibacterial resistance; Case report; GabonIntroduction
Global climate change may lead to the rise of pernicious human infections caused by environmental bacteria, such as Chromobacterium violaceum, normally infecting animals. Among the Chromobacterium genus, Chromobacterium violaceum (C. violaceum) is the only species that is known to infect humans [1-3].
Over the years, this facultative anaerobic Gram-negative environmental proteobacterium has been responsible for over 200 cases of human infection worldwide, mostly in Southeast Asia, Australia, Africa, and Florida. Despite the scarcity of C. violaceum infections, they can lead to severe sepsis with a high risk of mortality particularly in immunocompromised individuals (G6PD deficit, diabetes, lymphogranuloma, etc.,) who appear to be more susceptible to this bacteremia [4,5]. In humans, C. violaceum infections can be challenging to treat due to antibacterial resistance.
Case Presentation
Patient’s concerns and clinical findings: A 12-month-old female infant was admitted to the paediatric ward of the Centre Hospitalier Régional Amissa Bongo de Franceville (CHRAB) for a prolonged fever lasting seven days. This patient had a history of hospitalization. He had been treated for malaria and an ENT infection (details of ENT infection not at disposal at the time this report was written) following episodes of febrile attacks at 2 and 4 months after birth. The patient had one week’s history of fever before admittance to the paediatric ward. On admittance, the clinical examination was uneventful.
Primary diagnoses and interventions: The laboratory assessment highlighted the presence of an infectious syndrome: neutrophilia, a positive thick drop (parasitaemia not given here) and the C-reactive protein was 96 mg/L. The child has been put under injectable antimalarial treatment following national guidelines and probabilistic antibiotic therapy combining injectable ceftriaxone (C3G at a dose of 10 mg/kg/d for 10 days) with injectable Tobramycin (aminoglycoside at a dose of 3 mg/kg/d for 3 days) in direct intravenous. The clinical course of the malaria access was very satisfactory, but the infectious syndrome persisted. A CBEU carried out post-probabilistic antibiotic therapy came back positive and the child was switched to Cefaclor 125 mg/5 ml (oral C1G at a dose of 25 mg/kg per day).
The CBEU was performed as follows: a sample of urine was collected using a paediatric collection device under strict aseptic conditions. Following mixing, 10μL of urine was streaked onto a Cystine- lactose-electrolyte-deficient (CLED) agar medium and then, incubated at 37°C for 24 hours.
After 24 hours of incubation, whitish, smooth, shiny colonies appeared, catalase + oxidase +. The leucocyturia on the Kovaslide slide was around 30.103 leucocytes/μL. The urine germ count (UGC) was 104CFU/mL. Identification using the Vitek 2 automated system of bioMerieux (France) gave C. violaceum with 94% precision. Antibiotic susceptibility testing (AST) was performed using the Kirby-Bauer method also called the agar disc diffusion technique. The interpretation of AST was done according to CLSI recommendations and using the breaking points of E. coli (Table 1).
Table 1: Note: AMC: Amoxicillin/Clavulanic Acid; KF: Cefalotin; FOX: Cefoxitin; CTX: Cefotaxim; ETP: Ertapenem; NA: Nalidixic Acid; CIP: Ciprofloxacin; PRL/TZP: Piperacilin/Tazobactam; OFX: Ofloxacin; TOB: Tobramycin; TI: Ticarcilin; IMI: Imipenem; AK: Amikacin; STX: Trimethoprim/ Sulfametoxazol; CAZ: Ceftazidim; CN: Gentamicin; CRO: Ceftriaxon; S: Susceptible; R: Resistant.
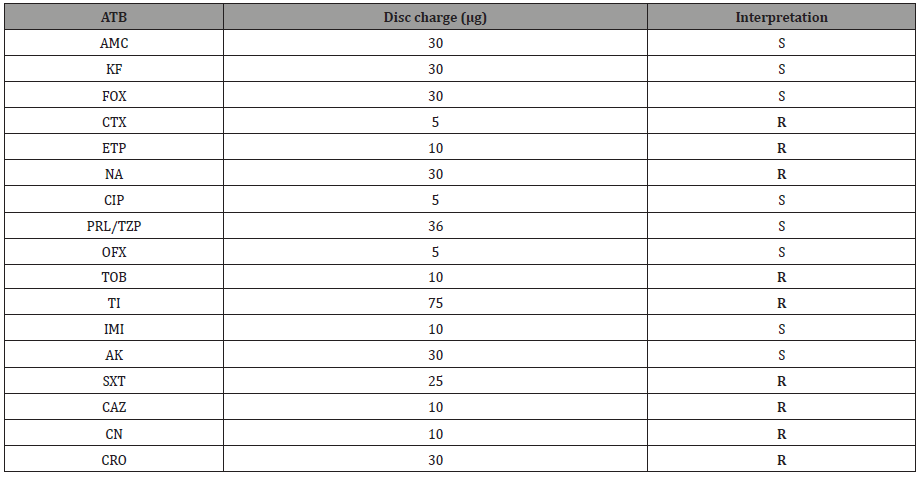
The results in Table 1 show that some antibiotics (AMC, KF, FOX, CIP, PRL/TZP, OFX, IMI and AK) could have been efficiently used against this strain of C. violaceum. The bacterium strain exhibited resistance to some antibiotics belonging to diverse families: TI, ETP, CTX, CAZ and CRO from the 3rd Generation Cephalosporins (C3G); CN and TOB from the Aminosides; NA from the urinary Quinolones; and STX from the sulfonamides.
Outcome: The infectious syndrome disappeared after the probabilistic treatment was adjusted post-CBEU and switched to Cefaclor 125 mg/5ml at a dosage of 25 mg/kg/day. The child was then released and was doing great by the time we wrote this report.
Discussion
Only one case of C. violaceum was reported in the region of Central Africa and the Great Lakes so far [6], making the case here the second and the very first in Gabon. While most pigmented strains of C. violaceum have been described to be pathogenic to humans, some non-pigment strains can express the same pathogenic power [7-11].
The persistence of the infectious syndrome after clearing the malaria infection and especially after receiving probabilistic antibiotic therapy (“shot” of Ceftriaxone and Tobramycin) seems to underline to failure of the medication. The bacterium C. violaceum was identified following the CBEU and its antibiotic susceptibility tests showed that this bacterial strain was resistant to Ceftriaxone, which could further explain the persistence of the infectious syndrome despite the use of this compound by the patient. This was observed by another research group which also revealed that C. violaceum was as well resistant to Tobramycin [12]. However, more recent work has confirmed these findings by demonstrating that several commonly used antibiotics are inactive against this bacterial species, namely Penicillins and Cephalosporins, but the mechanisms that may underline this resistance need to be further explored. It would though appear that this resistance is of an acquired type and runs counter to the observations of several authors [7,13].
The resolution of the infectious syndrome following the lab CBEU report and the administration of a C1G as a treating compound suggested that this drug was indeed active on C. violaceum. Though our results confirmed bacterial strain sensitivity, it is still quite puzzling as it is unusual [14]. The most active antibiotics belong to the Carbapenem (Imipenem) and Fluoroquinolone (especially Ciprofloxacin) subfamilies [13,7, 15]. Responsiveness to one Carbapenem and two systemic Fluoroquinolones has been frequently described by other authors [15] and of all the aminoglycosides tested; only Amikacin was found to be a good therapeutic choice to treat the infection, which is consistent with findings in the management of bacteremia due to this pathogen in Nepal [7].
Conclusion
C. violaceum appears to be an emerging pathogen whose most human infections are lethal. The scarcity of human infections due to this microorganism, particularly in Africa, contributes to the exacerbation of clinicians’ and laboratory staff’s lack of awareness of its real impact in case of misdiagnosis and late or inappropriate therapeutic management.
Our findings highlight the need for a ‘One Health approach’ to monitor and control the expansion of this mesophilic bacterium.
Author Contributions
The conception, study design, execution, acquisition of data, analysis and interpretation (or in all these areas) of the work reported were significantly made by all authors. They have drafted, and substantially revised the article.
Acknowledgement
Our thanks go to all the staff of the medical lab analysis of the Interdisciplinary Centre for Medical Research of Franceville (CIRMF) for allowing us to perform all the technical analyses of the patient sample.
Conflict of Interest
There are no conflicts of interest involving this article.
References
-
Romeo Wenceslas Lendamba*, Pierre Philippe Mbehang Nguema, Mesmin Yves Moussounda and Gaël NSI. First Reported Case of Human Infection with Chromobacterium Violaceum in Gabon: Antibiotic Susceptibility Patterns and Treatment Outcome. Online J Ecol Environ Sci. 1(3): 2023. OJEES.MS.ID.000511.
-
Chromobacterium violaceum, Cytobacteriological examination of urine, Cystine lactose electrolyte deficient, Leukocyturia, Antibacterial resistance, Gram-negative environmental proteobacterium, Antibiotic therapy, Ceftriaxone, Tobramycin, Ciprofloxacin
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






