 Research Article
Research Article
Surface Treatments of Titanium and Zirconia Implants: Impact on Bacterial Adhesion (Systematic Revue of Literature)
Moudni S1*, Moussaoui H1, Rochd T2, Boujoual I1 and Andoh A2
1Fixed Prosthesis Department, Faculty of Dentistry of Casablanca, Hassan II University of Casablanca, Casablanca, Morocco
2Rochd T, Biology and fundamentals department, Faculty of Dentistry of Casablanca, Hassan II University of Casablanca, Morocco
Moudni S, Fixed Prosthesis Department, Faculty of Dentistry of Casablanca, Hassan II University of Casablanca, Casablanca, Morocco.
Received Date: May 09, 2022; Published Date: May 20, 2022
Introduction
The use of dental implants to restore the loss of one or many teeth has become a widely used treatment option in daily practice. However, as soon as implant surfaces or their components are exposed to human oral cavity, they are immediately covered by an acquired film and instantly subjected to bacterial colonization. This is directly influenced by the surface properties of the materials, including chemical composition, surface roughness, surface energy... [1]. In modern biomaterial research, implant surfaces are primarily modified to increase bone integration into the alveolar bone. Recently, implant surfaces are also modified to reduce biofilm formation after exposure to the oral cavity [2]. Currently, many implant systems with different surface treatments are available on the market, which makes it difficult for the practitioner to choose. There are two main categories of implant surface treatments [3]:
-Either by adding substance: this is the addition treatment.
-or by altering the smooth surface: this is the subtraction treatment.
The main objective of this systematic review was to evaluate the impact of different surface treatments of titanium and zirconium implants on bacterial adhesion. The secondary objective was to compare bacterial adhesion on titanium and on zirconium implants.
Material and Methods
Research strategy
Three computer databases were used for the literature search: Pubmed, Cochrane, and Science Direct.
The search was conducted between 01/ 01/ 2011 and 01/ 02/ 2022. The review included well-conducted in vivo and/or in vitro trials and randomized trials written in English, evaluating the surface condition of titanium and zirconium dental implants and its relationship with bacterial adhesion. Exclusion criteria were case reports, systematic reviews of the literature, meta-analyses, literature reviews, animal and cadaver studies, and articles dealing with bacterial adhesion on supra-implant prosthesis.
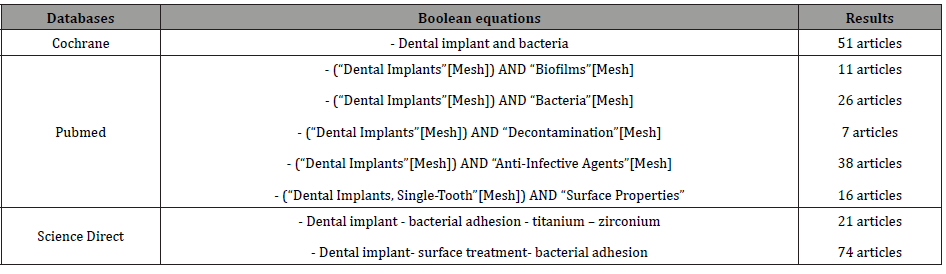
The table below represents the keywords used in the different boolean equations and for each database (Table 1).
Table 1: keywords and boolean equations.
Assessment of methodological quality
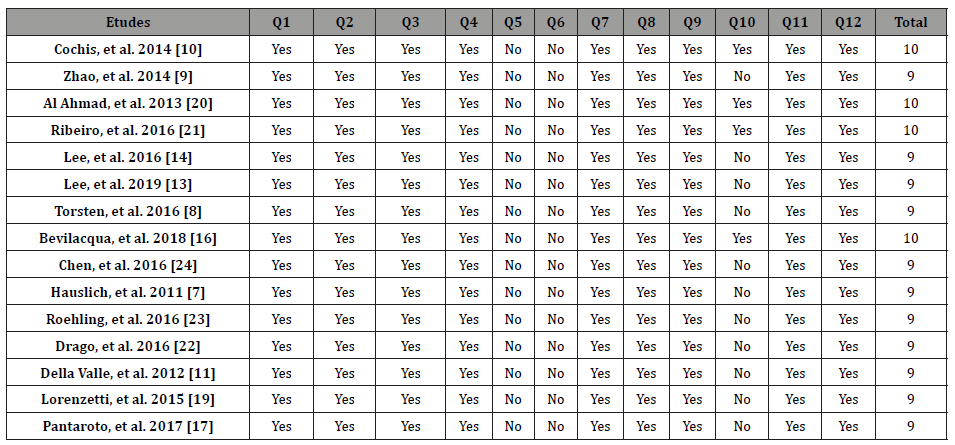
Two reviewers (M.S. & B.I.) assessed the methodological quality of the studies selected for analysis. This assessment was based on the tool “The Critical Skills Appraisal Program” (CASP) which was created from guides produced by the Evidence Based Medicine Working Group and published in the Journal of the American Medical Association [4].
Data extraction
To prepare and structure our systematic review, the focused
question was developed using the PICO criteria:
P: Participants => Titanium and zirconium dental implants
I: Intervention => Implants with different surface treatments
(by addition/subtraction) placed in contact with different bacteria.
C: Comparison => Control group (titanium or zirconium discs
without surface treatment)
O: Outcomes => Relationship between the surface
characteristics/the material of the implants and bacterial adhesion.
Data extraction was completed by 2 readers independently,
with formal processes for discussion and consensus building in
case of disagreement to minimize subjectivity during the multiple
stages of completion.
The writing of this systematic review followed the PRISMA
Statement “Preferred Reporting Items for Systematic reviews and
Meta-Analyses” Moher, et al. [5].
Results
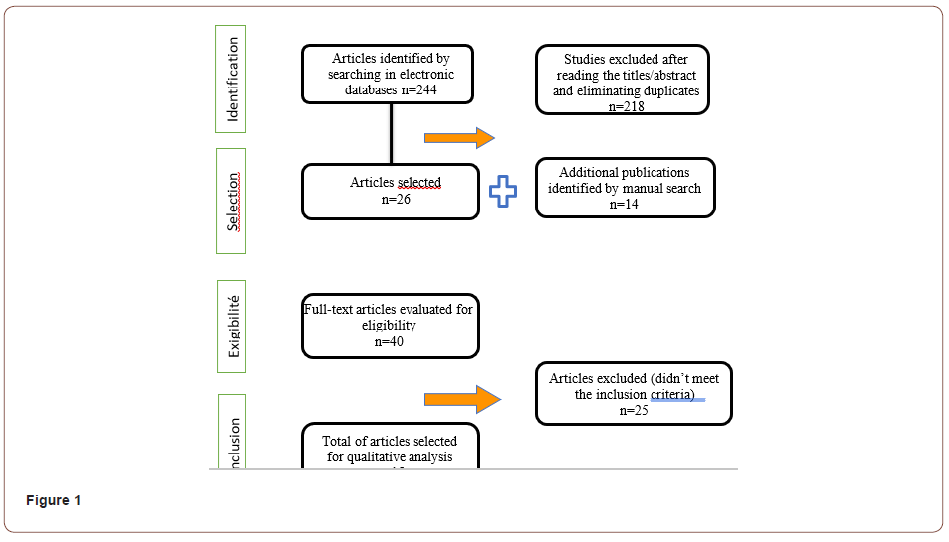
The search of the three databases identified a total of 244 studies from the automated search, from which we retained 26 studies after the first selection based on the reading of titles and abstracts and after eliminating duplicates. Through the manual search, we were able to group 14 articles. Thus, we had a total of 40 articles from which 25 articles were eliminated after applying the inclusion and exclusion criteria, resulting in the selection of 15 articles included in our review (Figure 1).
The 15 selected studies include 11 in vitro studies, 2 studies conducted in vivo and 2 studies conducted in vitro and in vivo. They were all performed using titanium or zirconium discs with different surface characteristics. The control group was constituted of titanium or zirconium discs without surface treatment. In vivo studies were performed with intra-oral splints with different discs for each tested sample.
Of the 15 studies included in our review, 10 investigated bacterial adhesion only on titanium discs. The other 5 studies included, in addition to titanium discs, zirconium discs with different surface treatments.
The different parameters measured were essentially surface roughness of the material, its wettability through the measurement of the contact angle, chemical composition of the surfaces and analysis and quantification of the bacteria adhesion to the different surfaces after bacterial culture.
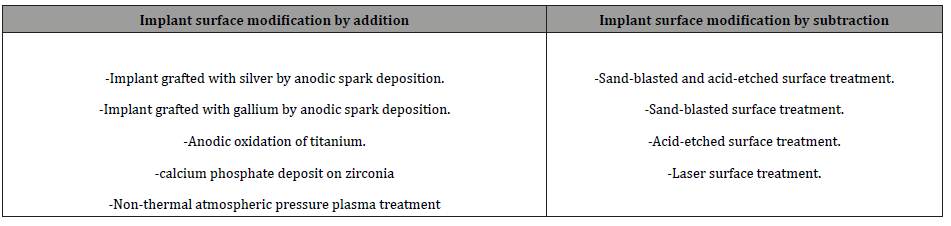
The different surface treatments used were multiple and heterogeneous and were classified into implant surface modification by addition or subtraction (Table 1) (Table 2).
Table 2: Classification of surface implant modifications used in the included studies.
Informations about the characteristics of these studies are presented in (Tables 3, 4), including a brief description of the studies and their conclusions and the quality assessment of included studies is summarized in (Table 5).
Table 3: Bacterial adhesion and implant surface modification by addition.
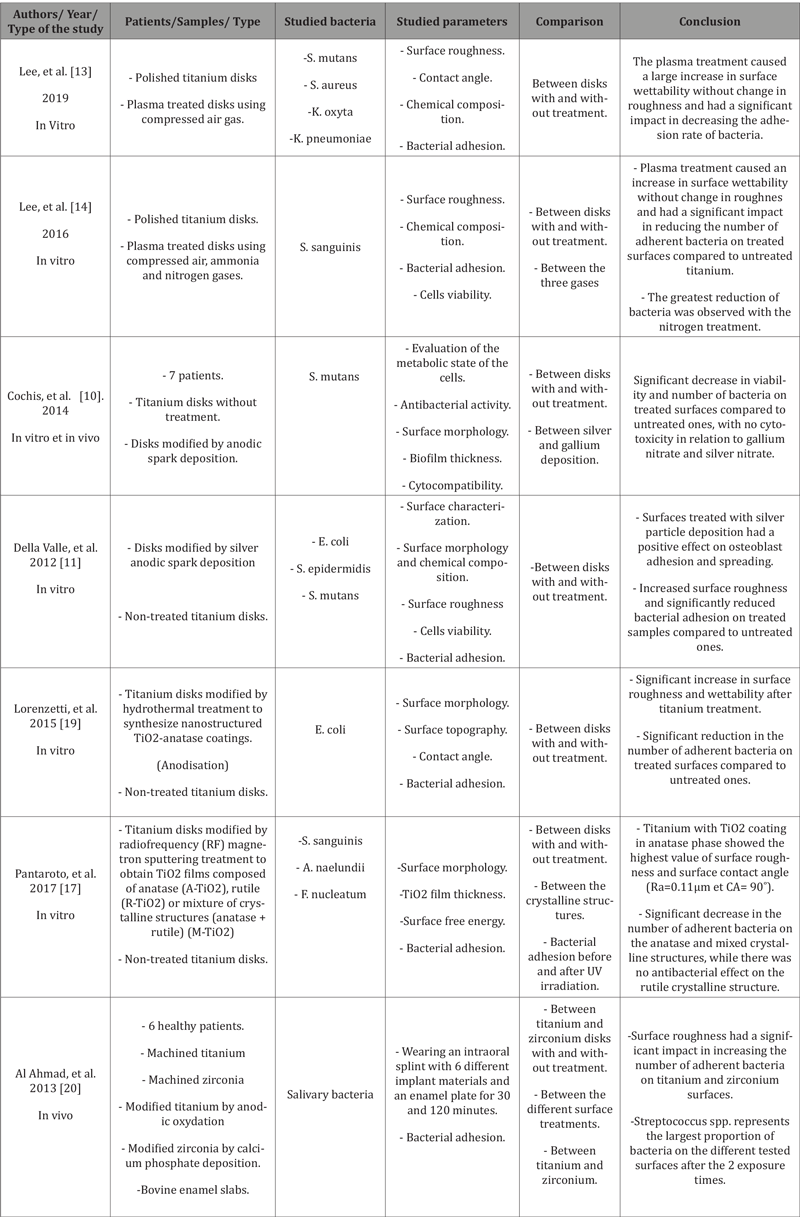
Table 4: Bacterial adhesion and implant surface modification by subtraction.
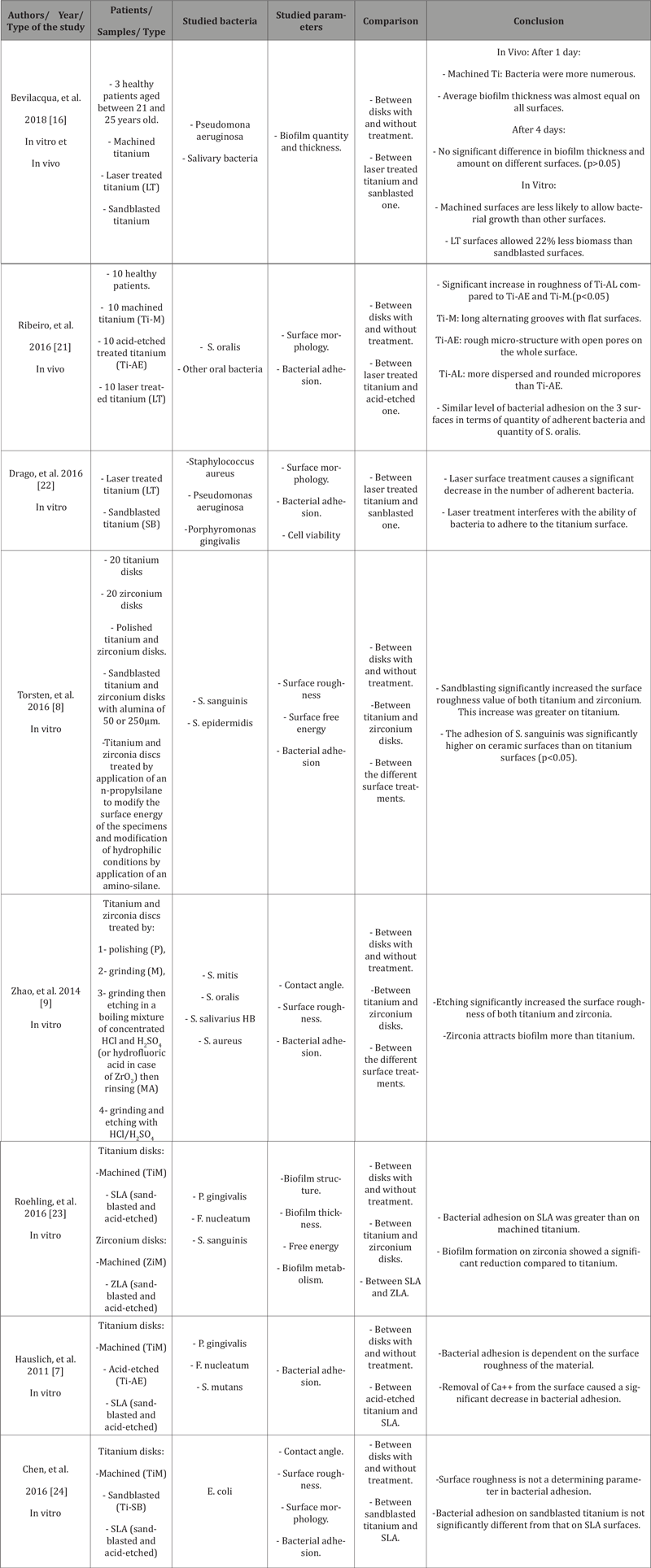
Table 5: Assessment of methodological quality of the included studies.
Discussion
This work focused on the effect of different surface treatments of titanium and zirconium dental implants on bacterial adhesion. This question was addressed by in vitro and/or in vivo studies. Several parameters are involved in the phenomenon of bacterial adhesion on dental implants. Surface roughness is a widely evaluated parameter. According to Wennerberg, et al. [6], dental implant surfaces are classified into four different groups based on their roughness: smooth surfaces (Ra < 0.5 μm), minimally rough surfaces (0.5 < Ra < 1.0 μm), moderately rough surfaces (1.0 < Ra < 2.0 μm), and rough surfaces (Ra > 2.0 μm).
According to Hauslich, et al. [7], increasing surface roughness increases bacterial adhesion to implant surfaces. However, other studies (12-20) have shown that surface roughness only affects the early stage of biofilm development. Torsten and Zhao [8, 9], on the other hand, have shown that roughness is not the only determinant of bacterial adhesion. Three-dimensional analyses of the microstructure are necessary [8]. Considering the studies carried out in vivo and in vitro using complex bacterial communities close to the oral context where the bacterial flora is rich and varied, it was concluded that the surface roughness intervenes only during the initial bacterial attachment.
Furthermore, the use of surface treatment by electro-deposition of silver or gallium showed a great decrease in bacterial adhesion on the surfaces despite the increase in surface roughness [10, 11]. This was explained by the fact that silver nanoparticles in the microporous titanium oxide layer are readily available to react with water and release silver ions. These later are known to bind strongly to electron donor groups in biological molecules containing sulfur, oxygen or nitrogen causing defects in the bacteria cell wall so that cell contents are lost. In addition, silver ions bound to proteins can alter bacterial cells metabolism and change membrane permeability and respiration. Both of these effects lead to bacterial cell death [9]. According to Wennerberg and Albrektsson [12], there is an optimal surface roughness window of 1 to 1.5μm. They consider that a higher value leads to a loss of bone anchorage. They add that it is very difficult to compare different studies, especially because the techniques used for characterization of surface topography vary considerably.
The surface energy of implant materials is also an important parameter influencing bacterial adhesion. Hydrophilic surfaces prevent bacterial attachment and can be achieved by increasing the wettability. The treatment of implant surfaces with plasma had led to significant increase in surface energy of titanium (4x more than untreated one), and consequently, to a significant reduction in the number of adherent bacteria [13, 14]. This was explained by the fact that bacteria with hydrophobic properties prefer hydrophobic surfaces and tend not to attach to hydrophilic surfaces and vice versa. However, the majority of bacteria involved in peri-implantitis are hydrophobic species. These results are in agreement with a review of literature about the wettability of dental implant surfaces, which concluded that among the benefits of hydrophilic surfaces is the reduction of adhesion of bacteria such as Staphylococcus aureus, Streptococcus sanguinis, and Staphylococcus epidermidis [15].
Concerning the crystalline phase of the surface, titanium dioxide can be found in three crystalline structures: anatase, rutile and brookite. These have a photocatalytic activity responsible for their anti-bacterial properties. The different structures can be generated by different methods like anodic oxidation, hydrothermal treatment and plasma treatment. TiO2-anatase coatings, which were previously proven to improve corrosion resistance, affect the plasma protein adsorption and enhance osteogenesis, have also shown strong antibacterial activity on titanium surfaces [16]. This can be attributed to the presence of anatase phase and its large band gap (3.2 eV) promoting high energy to create electrons and holes, and consequently to form more reactive oxygen species, when compared to rutile [17].
With regard to the implant material, titanium is the most frequently used reference material because of its biocompatibility and excellent mechanical properties. Recently, high-strength zirconia (ZrO2) implants have been invented as an alternative to titanium implants because of their resistance to corrosion and their enhanced aesthetics in case of exposure. Bacterial adhesion on titanium and zirconia does not seem to show any significant difference. Titanium is coated by a layer of surface oxide, which physical and mechanical characteristics are more closely related to ceramic than to metal. This phenomenon may explain why similar protein-binding properties on titanium and zirconium oxide have been reported and why zirconia did not show any reduced bacterial adhesion [8]. A randomized clinical trial, performed in vivo and in vitro, revealed no significant difference in the colonized surface area in the different discs (p=0.0730) as well as a high percentage of coverage by biofilm on all materials tested (90.9% of the total surface area of zirconia and 84.14% on machined titanium) [18]. Nevertheless, the number of studies on bacterial adhesion on zirconia remains very low, and other studies should be carried out on a larger number of patients to confirm the experimental results found [19-24].
Conclusion
Based on the results of this systematic review, the following conclusions can be drawn:
-Roughness is only involved in the early stage of biofilm development and not in its maturation.
-Increasing the wettability of materials by creating hydrophilic surfaces has shown very good results in reducing the number of bacteria adhering to them.
-The use of materials in anatase crystalline structure has proven to be an important factor in the development of an antimicrobial surface.
-The deposition of a thin layer of silver nanoparticles may be an option to provide the implant with anti-bacterial characteristics on its surface and contribute to the prevention of peri-implant inflammatory processes. However, it is essential that the particles are securely fixed to prevent their entrance to the circulation.
-As for adhesion on zirconia and titanium, the results of the majority of studies have shown no difference in bacterial colonization between the two. However, further clinical investigations are still needed.
Acknowledgement
None.
Conflict of interest
No conflict of interest.
References
- Teughels W, Van Assche N, Sliepen I, Quiryen M (2006) Effect of material characteristics and or surface topography on biofilm development. Clinic Oral Implants Res 17: 68-81.
- An YH, Friedman RJ (1998) Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res 43: 338-348.
- Martinez H, Renault P (2008) Les implants: Chirurgie et prothè Choix thérapeutique stratégique. Paris: CdP.
- Zeng X, Zhang Y, Kwong JS, Zhang C (2015) The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J Evid Based Med 8: 2-10.
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preffered reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4): 264-269.
- Wennerberg A, Albrektsson T (2009) Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res 20: 172-184.
- Hauslich LB, Sela M N, Steinberg D, Rosen G, Kohavi D (2011) The adhesion of oral bacteria to modified titanium surfaces: role of plasma proteins and electrostatic forces. Clinical oral implants research.
- Wassmann T, Kreis S, Behr M, Buergers R (2017) The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int J Implant Dent 3(1): 32.
- Zhao B, Van der Mei HC, Subbiahdos G, De Vries J, Rustema-Abbing M, et al. (2014) Soft tissue integration versus early biofilm formation on different dental implant materials. Dent Mater 30(7): 716-727.
- Cochis A, Azzimonti B, Della Valle C, Chiesa R, Arciola CR, et al. (2014) Biofilm formation on titanium implants counteracted by grafting gallium and silver ions. Wiley Periodicals.
- Della Valle C, Visai L, Santin M, Cigada A, Candiani G, et al. (2012) A novel antibacterial modification treatment of titanium capable to improve osseointegration. Int J Artif Organs 35(10): 864-875.
- Wennerberg A, Albrektsson T (2010) On implant surfaces: A review of current knowledge and opinions. Int J Oral Maxillofac Implants 25: 63-74.
- Lee MJ, Kwon JS, Jiang HB, Choi EH, Park G, et al. (2019) The antibacterial effect of nonthermal atmospheric pressure plasma treatment of titanium surfaces according to the bacterial wall structure. Scientific reports.
- Lee JH, Jeong WS, Seo SJ, Kim HW, Kim KN, et al. (2016) Non-thermal atmospheric pressure plasmafunctionalized dental implant for enhancement ofbacterial resistance and osseointegration. Dent Mater.
- Gittens RA, Scheideler L, Rupp F, Hyzy SL, Geis-Gerstorfer J, et al. (2014) A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater 10: 2907-2918.
- Bevilacqua L, Milan A, Del Lupo V, Maglione M, Dolzani L (2018) Biofilms Developed on Dental Implant Titanium Surfaces with Different Roughness: Comparison Between In Vitro and In Vivo Studies. Current Microbiology 75: 766-772.
- Pantaroto HN, Ricomini-Filho AP, Bertolini MM, Silva J HD, Azevedo Neto NF, et al. (2018) Antibacterial photocatalytic activity of different crystalline TiO2 phases in oral multi-species biofilm. Dental Mater 34(7): e182-e195.
- Rimondini L, Cerroni L, Carrassi A, Torricelli P (2002) Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int J Oral Maxillofac Implants 17: 793-798.
- Lorenzetti M, Dogsa I, Stosicki T, Stopar D, Kalin M, et al. (2015) The influence of surface modification on bacterial adhesion to titanium-based substrates. ACS Appl Mater Interfaces 7: 1644-1651.
- Al-Ahmad A, Wiedmann M, Fackler A, Follo M, Hellwig E, et al. (2013) In vivo study of the initial bacterial adhesion on different implant materials. Arch Oral Biol 58(9): 1139-1147.
- Ribeiro CF, Cogo-Müller K, Franco GC, Silva-Concílio LR, Campos MS, et al. Initial oral biofilm formation on titanium implants with different surface treatments: An in vivo study.
- Drago L, Bortolin M, De Vecchi E, Agrappi S, Weinstein RL, et al. (2016) Antibiofilm activity of sandblasted and laser-modified titanium against microorganisms isolated from peri-implantitis lesions. J Chemother 28(5): 383-389.
- Roehling S, Astasov-Frauenhoffer M, Hauser-Gerspach I, Braissant O, Woelfler H, et al. (2016) In Vitro Biofilm Formation on Titanium and Zirconia Implant Surfaces. Journal of Periodontology.
- Chen CJ, Ding SJ, Chen CC (2016) Effects of Surface Conditions of Titanium Dental Implants on Bacterial Adhesion. Photomed Laser Surg 34(9): 379-388.
-
Moudni S*, Moussaoui H, Rochd T, Boujoual I and Andoh A. Surface Treatments of Titanium and Zirconia Implants: Impact on Bacterial Adhesion (Systematic Revue of Literature). On J Dent & Oral Health. 5(4): 2022. OJDOH.MS.ID.000620. DOI: 10.33552/OJDOH.2022.05.000620.
-
Dental implants, Teeth, Oral cavity, Zirconium, Intra-oral splints, Dental implant surfaces, Staphylococcus aureus, Streptococcus sanguinis, Staphylococcus epidermidis
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






