 Review Article
Review Article
Present and Future Treatment Modalities for the Mitigation and Cure of Periodontal Diseases
Sandipan Mallick, Mrinmoy Barman, Sefali Halder Hota and Biswajit Mukherjee*
Department of Pharmaceutical Technology, Jadavpur University, Kolkata, India
Professor Biswajit Mukherjee, Department of Pharmaceutical Technology, Jadavpur University, Kolkata, 700032, India.
Received Date: September 11, 2023; Published Date: October 11, 2023
Abstract
Periodontal disease is a serious global health issue amongst people of all age groups. Non-surgical and surgical treatments, emerging and investigational therapies, lifestyle and systemic health management are amongst the available treatment strategies of periodontal diseases and disorders. However, certain lacunae are associated with these conventional treatment modalities. Conventional formulations available for managing periodontal diseases are tablets, hydrogels, films, wafers, fibre strips, dental devices, etc. These formulations are composed of antibacterial and antiinflammatory drugs. Chronic use of these formulations may lead to several adverse drug reactions. To overcome these drawbacks, advanced research is necessary for the development of non-invasive, cost effective, biocompatible, advanced technologies and regimen such as gene therapy, tissue engineering, nanotechnology, proteomics, vaccines, development of some novel dental formulations and some regimens for the mitigation and cure of several periodontal diseases. In this review, we elaborately discuss the clinical aspects of periodontitis and its available treatment modalities and management strategies with novel approaches for the development of modern therapeutic policies. They would lead to the opening of new avenues in the field of periodontology and provide better patient compliance.
Keywords:Gingivitis; Dental plaques; Adjunctive therapies; Host modulation therapy; Tissue engineering; 3D printing
Introduction
Periodontal disease is a serious worldwide inflammatory condition of the gum tissue gingiva, which affects the anatomy and physiology of the supporting pillars of a tooth, the periodontium (alveolar bone, connective tissue, ligament) and the gum itself [1]. Periodontal manifestations are seen in almost all age groups, although the severity, extent, sustenance, and percentages might vary globally [2, 3]. About 50-90% of the adult population worldwide are affected by periodontitis [4]. Microbial colonization forming dental plaques (biofilm), consisting of diverse, virulent micropathogenic species such as spirochetes, viruses, gram negative anaerobic bacteria are the driving forces behind the onset of periodontitis [5]. Intake of excessive sugar in the mouth leads to the interaction with the residing bacterial colonies that ferment them, thereby giving rise to several periodontal problems and tooth decay such as dental caries, toothache, and foul odor from the oral cavity (Halitosis). In addition to this, there are various other risk factors contributing to the pathophysiology of periodontal diseases. Accumulation and calcification of dental plaques over time, lead to the formation of deep periodontic pockets, crevices, and hard tartars between the margin of the tooth and the gum tissue, resulting in inflammation, swelling, bleeding, and loss of connective tissues, supporting bones, ligaments which cause weakening, decaying and loosening of the tooth [1].
Periodontal disease can be broadly classified into two main categories, that is, Gingivitis and Periodontitis, along with some other sub-categories based on clinical features and symptoms. Certain systemic disorders have been found to give rise to periodontal complications, such as diabetes [6], cardiovascular disease [7], respiratory [8] and Alzheimer’s disease [9]. As our understanding of the disease has evolved, so too have the treatment modalities employed by dental professionals. There are various treatment strategies available for periodontal therapy, ranging from surgical to non-surgical approaches, use of solid dosage forms, hydrogels, dental devices, films, and various other technologies and treatment policies. After the active treatment is completed, regular monitoring and supportive follow-up therapy are obligatory for the successful completion of periodontitis treatment [10]. Mechanical debridement, reimplementation of oral hygiene, and serious efforts for controlling and eradicating the probable risk factors of periodontal diseases are the pivotal tasks to be provided to the individual affected patients [11]. Although there are different types of treatment procedures available for the mitigation and cure of periodontal diseases, certain lacunae persist amongst the conventional therapeutic approaches. Conventional formulations available for managing periodontal diseases are tablets, hydrogels, films, wafers, fiber strips, dental devices, etc. These formulations are composed of antibacterial and anti-inflammatory drugs. Prolonged use of these formulations may lead to several adverse drug reactions. This review elaborately discusses the various aspects of periodontal diseases, their available treatment and management approaches, and the future perspectives of treatment modalities for the mitigation and cure of periodontitis.
Various types and causes of periodontal diseases
Periodontal diseases referred to as “gum diseases”, occur mainly due to several types of bacterial invasions inside the oral cavity, thereby leading to the inflammation and damage of the gum and associated tissues of the tooth. The formation of plaques and bacterial colonizations are the driving forces of periodontal disorders. Excessive intake of sugar leads to chemical interactions with the different residing bacteria in our mouth, resulting in the formation of thin bacterial films known as plaques which get converted into hard tartars if left untreated. Tartars become difficult to remove, and more and more plaques are formed leading to swelling, inflammation, and tremendous pain in the gum tissue gingiva. These, tartars, plaques, and the several types of bacteria aggravate over time and form deep periodontal pockets between the teeth and the gum tissue. As they get deeper, these pockets cause damage to the teeth, the gum, and the associated tissues and bones. The primary symptoms of periodontal aberrations include inflamed and unbearably painful tooth gum, changes in the color and texture of the gum and teeth from normal to bright red swollen appearance, foul smell (Halitosis) from the oral cavity, chewing and biting problems, loosened and soft teeth, bleeding of the gum tissue and pus formation. In addition to bacterial invasions, some other risk factors for periodontal infections include poor maintenance of proper oral hygiene, diseases directly affecting the body’s immune system such as AIDS and cancer, deficiency of proper dietary elements especially, vitamin C (ascorbic acid), diabetes and excess obesity, genetic predispositions, systemic and genetic disorders, smoking and chewing of tobacco, rheumatoid arthritis, hormonal changes and complications and socioeconomic status. Figure 1. illustrates the various probable risk factors of periodontal diseases.
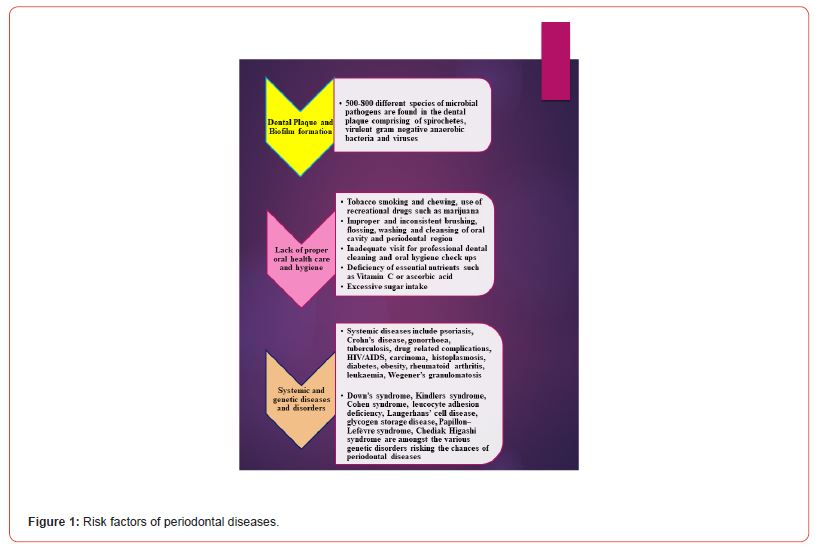
The various pathophysiological features of periodontal diseases can be described as follows:
Dental plaque: Both chronic gingivitis and chronic periodontitis are associated with the formation of dental plaques in the oral region [12]. Dental plaques comprise microbial biofilms formed by various pathogens. Several pathogenic species are found in human dental plaques [13]. Gram-negative anaerobic bacteria, spirochetes, and certain viruses are associated with the plaques for causing periodontitis, but no particular species is found to be solely responsible or most virulent for the onset of periodontal complications and hence, the entire biofilm is considered to be the causative force of periodontitis [14,15,16].
Microbial biofilm: Although the exact virulent pathogenic species of microorganisms responsible for periodontitis are not known, Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis are found to be associated with aggressive and severe or progressive periodontitis respectively [17, 18]. No particular pathogen species is known to differentiate between aggressive and chronic types of periodontitis in specific and both these types of periodontal diseases have been found to share a common microbial biofilm [19]. When human hosts are infected by viral pathogens, viral DNA or RNA are found inside the host tissues even after the symptoms of the infections have vanished and those viral infections may again arise at any point in time. Due to this, the association of pathogenic viruses with the onset of periodontal diseases is obscure [20].
Calcification [5]: The two major forms of dental plaques involved in periodontitis are the uncalcified supragingival form and the calcified subgingival form. The former one is prevalent on the oral and tooth surfaces, whereas the latter is mainly found between the gingival margin and the attachment point of the neck and root of the tooth. The subgingival calcified form, also known as, calculi, possesses a dark appearance and is difficult to eliminate from the oral cavity.
Immunopathogenesis: The pathophysiology of periodontal disease is not only due to the microbial biofilm formation but due to the imbalanced overreaction between the host immune system and the pathogens, known as dysbiosis [21,22,23]. There are significant and complicated variances between the pathogenic biofilm and the host defense mechanism [16, 24]. Due to this imbalance between the host immune system and the invaded microbial biofilm, inflammation and damage of various tissues are found to be associated with periodontitis [25]. Epithelial cells have been found to act as a hindrance to the invaded pathogens involved in periodontal diseases, thereby elevating both the innate and acquired immunity of the host [24].
Function of T cells: Host antibodies play a pivotal role for fighting against periodontal pathogens and the extent of their action is considered to be important in the context of periodontal diseases. B cells and lymphocytes are involved in the immune response against periodontal diseases. The contribution of T cells in cell-mediated immune responses is significant and they act by activating various T-helper (TH cells) responses such as TH1, TH2, and TH17 cells, although their extent of role is not clear yet. TH1 cells are found to be active during the initial stages of chronic periodontitis, whereas TH2 cells show their action during the following stages [26]. TH9, TH17, and TH22 subsets of regulatory T cells (Treg), other subsets of T-helper cells (TH Cells), and some cytokines such as interleukin 17 (IL-17) have been found to have effects on the immuno-pathophysiology of periodontitis [27].
Genetic predispositions: Thorough research has shown that different varieties of genes and polymorphisms are involved in the pathophysiology of periodontal diseases and the genotypes of periodontitis differ across individuals. Extensive studies have been made to figure out the genetic polymorphisms involved in cytokine production [28]. Several types of environmental factors are linked with the changes in gene expression. Alterations in the sequence, arrangement and characteristics of gene expression by epigenetic factors may have adverse effects on the functions of the genetic code [29]. Epigenetic research is a new area of modern research and extensive studies are being done to find the correlation between genes, genetic polymorphisms involved in periodontal diseases, and the environment.
Figure 2 represents the transformational changes of a healthy tooth into the periodontic state.
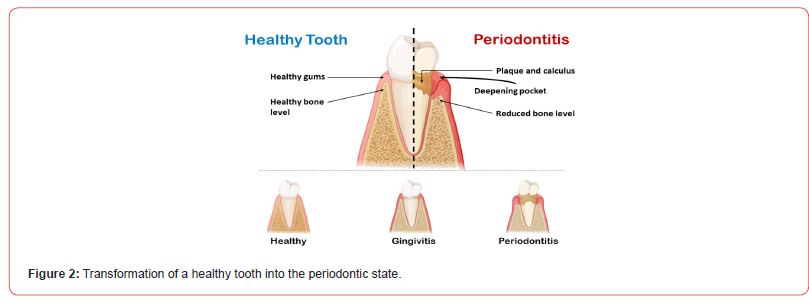
The following table describes the different types of periodontal diseases with their distinctive features and complications:
Gingivitis
It refers to the reversible inflammatory state of the gum tissue gingiva [5].
Gingivitis can be further classified into the following subcategories:
a) Necrotizing ulcerative gingivitis and periodontitis: Pathogens such as fusiform bacilli, spirochetes, viruses, and particularly virulent anaerobic microbes develop the biofilm [30] responsible for these forms of periodontal diseases. These types are acute, rare, and prevalent among individuals with weakened immune systems [5].
b) Peri-implantitis: This type of gingivitis targets dental implants, resulting in bone loss, thereby causing inflammation, swelling, and loosening of teeth [5].
Periodontitis
a) Aggressive Periodontitis: Localized or generalized are the two probable forms of this type of periodontitis and both of them are responsible for the early onset of a chronic state of periodontitis, symptoms being prominent between puberty and the early third decade of life [31].
b) Chronic Periodontitis: This stage of periodontal disease is characterized by a chronic inflammatory condition of the gum and other associated tissues of the periodontal region, causing irreversible loss of the epithelial cells, bone, and ligament, weakening the gingiva and thereby leading to soft and loosened teeth [5]. Syndromic chronic periodontitis, a type of chronic periodontal infection, having a background of severe genetic mismanagements, is a manifested result of few systemic diseases. This category of periodontitis might have major adverse effects on the host’s defence mechanisms. Various systemic and genetic disorders are associated with periodontal diseases which have been mentioned above in Figure 1.
In a study, Haubek et al. [17] revealed that the microorganism Aggregatibacter actinomycetemcomitans is associated with the formation of colonies in the periodontal region that cause aggressive periodontitis. Likewise, in another study, Amaliya et al. [15] discussed the effect of the microbial species Porphyromonas gingivalis in the context of chronic periodontal disease. Mombelli et al. [19] in their systemic review concluded that both the aggressive and chronic forms of periodontal diseases cannot be differentiated based on specific pathogenic invasions. However, the microbial biofilm forming dental plaques is found to be common in both cases. Again, as Kinane et al. [5] studied, pathological and aetiological features are the same in the case of both the aggressive and chronic forms of periodontitis and neither of these two types can be specifically distinguished based on biomarkers. Aggressive periodontitis is associated with inherited genetic predispositions and deformities. Elderly people are usually affected by chronic periodontitis, whereas, aggressive periodontitis has seldom clinical manifestations in children and adolescents.
Current treatment strategies of periodontal diseases
There are various treatment regimens available for periodontal management. They are described as follows:
I. Non-surgical treatments
A. Oral hygiene and preventive measures
It has been proven that plaque accumulation causes gingival inflammation and that plaque clearance reduces inflammation [32]. As a result, patient plaque removal is a critical component of nonsurgical therapy. Toothbrushing, interdental cleaning, and chemical plaque reduction are all parts of it.
Toothbrushing is a very common practice for oral hygiene. It should be done twice a day. It is a very effective and easy way to remove plaque from teeth. A toothbrush should have the following ideal characteristics:
(i) A handle that is appropriate for the user’s age and dexterity;
(ii) A head that is appropriate for the proportions of the patient’s mouth;
(iii) The polyester filaments that are not more than 0.009 inches in diameter;
(iv) Must have soft bristle configurations; and
(v) Bristle patterns that enhance plaque removal in the approximal spaces and over the gum line be used.
The optimum brushing time is two minutes for plaque removal. The frequency of brushing may be increased and toothpaste containing fluorides is recommended. Due to the recent developments in toothbrush design and technology, electric toothbrushes have evolved which are more effective in removing plaque in less time than normal brushing. Today there are various kinds of electric toothbrushes available in different sizes and shapes of brush heads. One can choose according to their choices and features [33].
B. Professional dental cleanings
Professional dental cleaning comprises scaling and root planing for removing dental plaque and calculus located above and beneath the gum line. Dental professionals provide advice to patients on how to improve their oral hygiene at their home. Following the cleaning session, the individual should return to the dental professionals for a reevaluation of the periodontal condition, which includes a physical examination that observes the state of the periodontium and measures probing depths to determine whether or not the disease process has been stopped. If the issue has been resolved, the patient should keep going to the dentist for frequent cleanings, as periodontal disease is a chronic condition that can recur if the right conditions are not met [5, 1, 34].
C. Antimicrobial agents and adjunctive therapies
Antibiotics can be provided both locally as well as systemically in situations of persisting periodontal disease that are resistant to non-pharmacologic therapy, based on the seriousness of the disease.
Chlorhexidine gluconate is an antibacterial agent that is commonly used in conjunction with mechanical periodontal therapy. It is commonly used as a mouth rinse, although it can also be applied as a gel, varnish, and subgingival chip. Using chlorhexidine, along with regular toothbrushing, can minimise dental plaque buildup and hence be quite successful in the cure of chronic periodontitis. A recent advancement in periodontal disease therapy was made which involves inserting a chlorhexidine gluconate chip into the periodontal pocket following cleaning and provides long-term, sustained chlorhexidine gluconate release into the infected site [35, 36].
Another alternative is an adjunctive therapy using an antibacterial agent after mechanical periodontal therapy. It is made up of minocycline hydrochloride microspheres that are inserted into the surrounding pockets after mechanical debridement. Its impact is similar to that of the chlorhexidine chip in that it successfully lowers tooth plaque buildup [37].
Some widely used antimicrobial drugs are penicillins, tetracyclines, quinolones, macrolides, cephalosporins, and nitroimidazole compounds. These antimicrobial drugs have different modes of action and they can be safely administered to patients having a wide range of susceptible microorganisms. Also, these agents could be used individually or in combination to widen their applications [38, 39, 40].
D. Host modulation therapy
Host modulation refers to the management of the inflammatory or immunological response in order to treat disease. The host response is greatly effective in controlling the periodontal disease by releasing inflammatory mediators (IL-1 and PGE2). These mediators attract pro-inflammatory cells like neutrophils and macrophages, that damage to the tissue matrix via the matrix metalloproteinase group of proteolytic enzymes (MMPs) [41]. Host modulation therapy has been used as a substitute to standard periodontal therapy. It consists of three approaches:
(i) The anti-inflammatory activity of antibiotics,
(ii) The use of nonsteroidal anti-inflammatory medications (NSAIDs), and
(iii) Bone-sparing pharmaceuticals.
Tetracycline has been widely tested and has proven antiinflammatory results in animal and clinical research models on periodontal disease which lowers the collagenolytic activity [42]. Similar effects are also observed in doxycycline and minocycline. Sub-antimicrobial doxycycline doses (SDD, 20mg twice a day), which is less than the minimum inhibitory concentration (MIC) can be used for anti-inflammatory action, has been found effective over conventional therapy [43, 44].
Prostaglandins are arachidonic acid derivatives that function as important inflammatory mediators, and prostaglandin E2 (PGE2) levels in gingival crevicular fluid (GCF) have been linked to enhanced attachment loss [45]. By blocking the cyclooxygenase pathway, NSAIDs prevent prostaglandin formation. Several studies have found that there is reduced alveolar bone loss and gingival irritation when compared to control groups [46, 47, 48]. However, widespread use of NSAIDs has not been observed, probably due to a lack of clinical benefit. There have been instances of little effect or negative effects that exceed the benefits.
Bone-sparing medications are being used to reduce bone resorption by decreasing osteoclastic activity. MMPs and PGE2 are both important activators of bone resorption, thus blocking them may help to prevent bone loss [49]. Bisphosphonates are another form of bone-sparing medicine that has been used as a potential adjunct to non-surgical treatment [50].
II. Surgical Treatments
A. Flap surgery and pocket reduction
Flap surgery, a crucial intervention in treating moderate to severe periodontal disease, addresses deep gum pockets, inflammation, and bone loss. The procedure involves assessing the patient’s condition through examinations and X-rays, administering local anesthesia for comfort, and gently lifting the gum tissue (flap) to access the roots and bone. This allows for the removal of plaque, tartar, and infected tissues, followed by smoothing the root surfaces to discourage bacterial adhesion. In the cases with bone damage, resective bone surgery may be necessary. The procedure concludes with repositioning and suturing the gum tissue. Postoperatively, patients can expect some discomfort, but diligent home care and follow-up appointments are vital for successful healing. Flap surgery offers benefits like reduced pocket depth and enhanced oral hygiene access, though it demands surgical skill and may yield variable responses [51]. Clinical evidence supports its effectiveness in preventing disease progression and tooth loss. Innovations in minimally invasive techniques and technology integration are advancing flap surgery, while patient education and informed consent play essential roles in preparing individuals for this treatment. Real-life cases demonstrate the positive impact of flap surgery on oral health outcomes [52].
B. Bone grafts and guided tissue regeneration
Bone grafts and guided tissue regeneration are critical components of periodontal therapy, particularly in the cases of significant bone loss. Bone grafts involve the surgical placement of bone or bone-like materials in areas where bone support has deteriorated due to periodontal disease. This procedure aims to stimulate new bone growth and improve the overall structure of the jawbone. Guided tissue regeneration, on the other hand, focuses on restoring not just bone but also other supporting tissues like the periodontal ligament and gum tissue. It involves the implementation of barrier membranes to specifically promote the growth of targeted tissues while inhibiting the invasion of undesirable ones. Both approaches offer promising solutions for the preservation of tooth-supporting structures and improving the long-term stability of teeth in patients with advanced periodontal disease [53].
C. Gum grafts and soft tissue augmentation
Gum grafts and soft tissue augmentation are essential procedures in periodontal care, primarily used to treat gum recession and improve asthetics. Gum recession exposes tooth roots and can cause tooth sensitivity and cosmetic concerns. Gum grafts involve surgically transplanting tissue, often from the patient’s palate or a tissue donor source, to cover and protect exposed tooth roots [54]. This procedure helps reduce sensitivity, enhance gum health, and restore a more natural gum line appearance. Soft tissue augmentation, a related technique, focuses on enhancing the volume and contour of the gum tissue to improve overall smile aesthetics [55]. These procedures contribute significantly to both oral health and the appearance of the smile, ensuring a more functional and pleasing outcome for patients.
D. Laser therapy in periodontics
Laser therapy has emerged as an innovative and minimally invasive technique in periodontics. It offers precise and efficient treatment for various periodontal conditions. Lasers, such as diodes or erbium lasers, are used to remove infected or damaged gum tissue, eliminate bacteria, and aid in the regeneration of healthy gum and bone tissue. Laser therapy typically involves less discomfort, swelling, and bleeding compared to traditional surgical methods. Additionally, it can often be performed with minimal or no anesthesia. While it shows promise in treating periodontal disease, its effectiveness varies depending on the specific condition and patient. Nevertheless, it represents a notable advancement in the field, providing an alternative option for patients seeking less invasive periodontal treatments [56-58].
III. Emerging and investigational therapies
A. Microbiome-based therapies
Microbiome-based therapies are a promising frontier in periodontal care, capitalizing on our growing understanding of the oral microbiome’s role in gum health. These therapies aim to rebalance the oral microbiota, fostering a healthier bacterial composition and thereby mitigating the progression of periodontal diseases. Approaches include probiotics, prebiotics, and targeted antimicrobial agents that selectively disrupt harmful bacteria while preserving beneficial ones. By targeting the root cause of gum disease-microbial dysbiosis, these therapies hold the potential for more effective and tailored treatments, potentially reducing the reliance on antibiotics and invasive procedures in the management of periodontal conditions [59, 60]. Ongoing research in this field continues to uncover promising avenues for microbiome-based interventions in the fight against gum disease.
B. Stem cell therapies
Stem cell therapies are at the forefront of innovative periodontal treatments, harnessing the regenerative potential of stem cells to restore damaged gum and bone tissues. These therapies entail the harvest and use of an individual’s mesenchymal stem cells that may be differentiated into a variety of cells, including those required for tissue repair. Stem cell-based treatments are particularly promising for regenerating lost periodontal tissues and promoting long-term periodontal health [61-63]. While research and clinical trials are ongoing, these therapies represent a promising avenue for enhancing the outcomes of periodontal procedures and may revolutionize the field by offering regenerative solutions for severe periodontal conditions.
C. Immunomodulatory approaches
Immunomodulatory approaches in periodontics focus on modulating the host immune response to better manage periodontal diseases. These therapies aim to balance the immune system’s reaction to bacterial pathogens in the oral cavity. They may involve medications, biologics, or other interventions that regulate inflammation and immune cell activity. By controlling the exaggerated immune response often seen in chronic periodontal conditions, immunomodulatory approaches seek to mitigate tissue damage and promote a healthier oral environment [64]. While research in this field continues, these approaches hold promise for enhancing the effectiveness of traditional periodontal treatments and improving long-term outcomes for patients with gum diseases.
D. Precision medicine in periodontal disease
Precision medicine in periodontal disease represents a tailored approach to treatment based on individual patient characteristics. This emerging field takes into account genetic, environmental, and microbial factors to customize therapeutic strategies. Through genetic testing and advanced diagnostics, clinicians can identify patients’ susceptibility to specific forms of periodontal disease and tailor interventions accordingly. Precision medicine not only enhances treatment effectiveness but also minimizes the trialand- error aspect of periodontal care. By focusing on the unique genetic and biological profiles of patients, it holds the potential to revolutionize the management of periodontal disease, offering more targeted and efficient therapies while improving overall oral health outcomes [65, 66].
IV. Lifestyle and systemic health management
A. Smoking cessation
Quitting smoking is an important element of periodontal disease treatment [67]. Smoking cigarette is a common cause for gingivitis because it suppresses the immune system’s reaction to bacterial infection, reduces blood flow to the gums, and hinders tissue repair. It is critical for optimal treatment outcomes to encourage people with periodontal disease to quit smoking. Quitting smoking not only enhances the efficacy of many periodontal therapies, but it also lowers the risk of disease progression and tooth loss [68]. Dental practitioners play an important role in helping patients quit smoking and improve their overall dental and systemic health by providing assistance, tools, and counseling.
B. Nutritional considerations
Nutritional considerations play a significant role in periodontal health. A balanced diet rich in essential nutrients, particularly vitamins C and D, antioxidants, and omega-3 fatty acids, can support gum health and overall oral hygiene. These nutrients aid in tissue repair, reduce inflammation and strengthen the immune system’s response to infections. On the other hand, a diet high in sugars and processed foods can contribute to gum disease by promoting bacterial growth and inflammation. Educating patients about the impact of nutrition on their oral health and encouraging healthy dietary choices can complement periodontal treatment and promote long-term gum wellness [69, 70].
C. Diabetes management and periodontal disease
Managing diabetes is crucial in the context of periodontal disease. Diabetes and gum disease have a bidirectional association since uncontrolled diabetes can increase periodontal difficulties and uncontrolled gum disease can make it difficult to maintain blood sugar levels [71]. Effective management of diabetes, including monitoring blood glucose levels and adhering to prescribed medications or insulin regimens, is vital to mitigate the risk and severity of periodontal disease [72]. Conversely, addressing periodontal disease through good oral hygiene and dental treatments can help improve glycemic control in diabetic individuals [73]. Thus, coordinated care between healthcare providers and dental professionals is essential for patients with both conditions, as it can lead to better overall health outcomes.
D. Cardiovascular health and periodontitis
The relationship between cardiovascular health and periodontitis is increasingly recognized. Periodontal disease has been associated with a higher risk of cardiovascular conditions like heart disease and stroke [74]. The mechanisms linking the two include systemic inflammation and the spread of oral bacteria into the bloodstream. Managing periodontal disease through effective dental treatments and good oral hygiene practices may help reduce this risk. Furthermore, individuals with cardiovascular issues should be aware of the potential impact of gum disease on their overall health and work with their healthcare providers to address both oral and heart-related concerns for better systemic health outcomes.
Future perspectives of periodontal treatment advancements and management
Conventional formulations available for managing periodontal diseases are tablets, hydrogels, films, wafers, fibre strips, dental devices etc. These formulations are composed of antibacterial and anti-inflammatory drugs. Chronic use of these formulations may produce adverse drug effects. To overcome these problems some modern approaches are necessary. Some of the modern techniques to treat the dental inflammations are host modulation, scaffolding, gene therapy, tissue engineering, antiviral therapy, vaccination, proteomic therapy, nanoparticle therapy and 3D printing.
Drugs are used mainly anti-inflammatory and anti-bacterial which can give localised relief for short term, but these above technologies are used for the treatment of long term benefits and patient compliance.
Several modern approaches and researches are going on to control the chronic periodontal diseases. Those are discussed below:
a) Host modulation
Host modulation becomes an important technic for the treatment of periodontal diseases which reduce the dental biofilm, the main cause of periodontal diseases. As the host response vary with the drug, host modulation has become an important tool to control and regulate the immune system of the host for effective long term treatment. It can help to balance the anti-inflammatory factors and mediators [75].
The treatment of Grade B Stage 2 periodontitis has been easier with host modulation with reduced dental plaque and debris formation, therefore infection can be treated with anti-bacterial drugs for a longer period of time [76, 77].
The common drugs for the treatment of these kinds of diseases are antibiotics such as tetracyclines, doxycycline and NSAIDs (non-steroidal anti-inflammatory drugs), such as ibuprofen, aspirin. Some bone sparing drugs such as bisphosphonates which are used to conjugate with hydroxyapatite crystals and interact with osteoclastic activity can also be used in periodontal diseases to prevent bone loss. These drugs can produce several adverse reactions and withdrawal of these drugs may reduce the therapeutic efficacy. If these drugs are used with specialized host modulating agents like some lipid mediators or omega 3 poly unsaturated fatty acid, the therapeutic efficacy can improve with manifold. For example, Resolvins have been used with these mediators for treating periodontal disease for prolonged time and given satisfactory results. [78, 79]. Aspirin also when combined with these mediators, increases the production of resolvin which show to control the periodontics and regulate the bone growth and regeneration [80].
So, the studies show that host modulation therapies with locally administered anti-inflammatory drugs have been a useful tool for the future prospect of periodontic diseases and its therapy.
b) Scaffolding matrices
Scaffolds are also useful for the treatment of periodontal tissue growth and regeneration. Scaffolds can be used for controlling many different areas [81]. As it is the main matrices, many mediators can be incorporated to these scaffolds for tissue growth and regeneration. For example, the controlled release strategies of the drugs and the mediators can be achieved by mixing the bioactive factors with matrices or entrapping them within gel matrices or within hydrophobic micro particles. They can also be bound with the active sit or the covalently bound with the matrices. For example, [82] a polyethylene glycol-based hydrogels as BMP (bone morphogenetic proteins)-2 can be used as delivery vehicles in critical sized calvarial defects.
c) Gene therapy
Gene therapy can also be another important tool as human gene can be modified by a special genetic expression therefore controlling the tissue growth of a particular organ. Similarly, with this gene therapy by modifying the expression, the periodontal diseases can also be controlled. The damaged portion of the periodontic tissues can be regenerated by tissue regrowth. Transferring the specific genetic expression to the DNA of a cell can change the protein formation of the cell, and cell signalling, therefore, tissue repair, regrowth and defect correction can be done by these techniques. Bubble liposomal delivery to the plasmid DNA of the gingiva in an animal study showed the way for targeted tissue therapy [83].
Therefore, the gene therapy and gene activated matrix can be useful in the treatment of regeneration of tooth-supporting structures by supplying the specific genetic code as the growth factors. Gene activated matrix can act as a local gene depot to supply the required genetic expression and favouring the growth factors for tissue growth [84].
The various gene therapies stimulate the periodontium tissue which express the proteins (for specific tissue growth and protein engineering) TGF, BMP-2, 6,7,12, bFGF, VEGF, and PDGF. Research shows these proteins can encode the non-osteogenic fibroblast, myoblast osteoblast etc. for periodontic tissue growth in vitro as well as in vivo gene transfer. Cell culture is used in different in vivo gene transfer experiments. BMP-7 and BMP-9 become the most effective for tissue growth of cells with an adenoviral infection and they can perform cell differentiation for oestrogenic cells [85].
Gene therapy can also be applied as a challenging field in engineering bacteriophages to treat the several pathogens of periodontal infections. Bacteriophages can destroy the infective bacteria of the periodontic sites as they contain protein structure with DNA [86].
d) Periodontal tissue engineering and bone regeneration (PTEBR)
Periodontal tissue engineering and bone regeneration is another intervention in grafting the periodontal ligament, cementum and alveolar bone surrounding teeth, where ridge reconstruction is essential part of the treatment for future implant. Sinus floor augmentation and regeneration of peri-implant osseous defects are based on this PTEBR. To withstand the biomechanical forces from mastication and to restore the normal life the technic of periodontal regeneration and cementogenesis on the root surface and oblique insertion of periodontal ligament fibers have become a very important step [87]. Periodontal tissue engineering and bone regeneration (PTEBR) has the objective to cure the permanent damage and defects of the periodontal region, therefore help to cure any periodontal inflammatory diseases due to these defects. PTEBR is combined with the other additional field for support such as cell signalling, scaffolds, and gene therapy.
e) Antiviral agents
There are several viruses available in the periodontal biofilm responsible for pain and tissue inflammation. For example, Epstein- Barr virus (EBV), cytomegalovirus and herpesvirus are some of the important viruses which cause the severe implications of periodontal diseases. Systemic antiviral drugs are useful for their treatment. Valacyclovir is used for the effective treatment of EBV and cure the clinical manifestations due to the periodontal lesions. Valacyclovir (Valtrex, GlaxoSmithKline) if intervened with nonsurgical full-mouth debridement can give better result and clinical improvement in Grade B Stage 2 periodontitis. It reduces the viral load and cure the sores, pain and discomfort to the gingiva. It works by suppressing viral multiplication and helps in healing sores to relieve pain and discomfort. Acyclovir can also be used to treat the lesions of herpetic infection and in oral lesions of HIV seropositive patients.
Antiviral therapy is useful in the lytic stage of the viruses and there are huge sources of latent viruses in periodontal infection [88, 89].
So, in many resistant, unremitting periodontitis, antiviral therapies along with mechanical debridement have become the rational treatment. Many antiviral mouth rinses are therefore under clinical trial as therapeutic agents.
f) Vaccines
Vaccines are used for the preventive measurement of any diseases. Therefore, for the prevention of periodontal diseases, researches are going on to formulate some vaccines also. The most common periodontic pathogen is Porphyromonas gingivalis, a Gramnegative bacterium which has been targeted for the preparation of vaccines. The whole cell body or its several antigenic parts is/are taken as target for vaccines production. The different antigenic parts of the microbes, which are taken for vaccines production precursor, are bacterial fimbriae, lipopolysaccharide and its polysaccharide capsule [90, 91]. Vaccines are used for active immunisation of host having possibilities of periodontal infections. After vaccination, the host body will become permanently immunised from this type of infections and its manifestations.
The researches are under trial. If the research becomes successful then it would be a long term benefit for the society in all over the world.
g) Proteomics
Tertiary proteins arranged in a specific 3D shape are the most important biological elements. A proteome is the 3D, alfa helix structure of protein and proteomics refers to the formulations of the protein which can be used for several physiological activities. Periodontal pathogens secrete several enzyme secretions which can be analysed to identify those pathogens. Several bio protein markers are used to analyse the physiologic effect of the secretary enzymes of the periodontal pathogens. The pathogens like Fusobacterium nucleatum and P. gingivalis form the biolfilm to the periodontal region and the specific protein biomarker helps to identify them. Salivary visfatin is a potential inflammatory salivary biomarker responsible for the pathogenesis of periodontitis [92]. For identification of gingivitis and periodontitis, Azurocidin is another potential biomarker candidate. Gingival crevicular fluid proteome is the main source of them [93]. Biomarkers are very much useful to detect the intensity of the infections, inflammation and deterioration due to the diseases.
In Grade B Stage 2 periodontitis, the tissue destruction is due to the protein cleavage in hostgum due the secretion of a proteases, known as gingipains by the organism, P. gingivalis. The molecular mechanism behind the tissue destruction can easily be diagnosed by the proteomics biomarkers. Gingipains now therefore become the point of interest of research for the prevention of periodontal infections. Studies are going on to examine the gingipain structure and its affinity and biocompatibility [94, 95].
Therefore, proteomics has become a challenging field in periodontal diagnostics and treatment.
h) Nanoparticles
Nanoparticles can act as promising vehicles for targeted and localized delivery of chemotherapeutic agents for the treatment and management of periodontal diseases. In addition to this, milestones in periodontology such as regeneration and repairment of damaged tissues, bones, ligaments and other parts of the diseased periodontium can be achieved with the help of nanotechnology. Research shows that hydrogels composed of nanomaterials such as nanofibers possess the uniqueness of exhibiting anti-oxidant, anti-inflammatory and anti-microbial properties against the virulent pathogenic species of periodontal diseases. Drug-polymer conjugated nanoparticles have been found to be effective in curing periodontitis, as they maintain a constant high level of drug concentration inside the crevices and deep pockets formed by the periodontal pathogens [96, 97]. Nanoparticles can load large number of molecules in them due to their surface-volume ratio in a single go, ensuring targeted, localized, sustained and controlled delivery of cargos to the affected cells and tissues of the body, thereby reducing the probabilities of drug-drug interactions, multi drug resistance, drug related systemic side effects and toxicities. Not to mention, enhanced bioavailability of drug molecules, prolonged duration of drug action, mucoadhesiveness, stimuli responsive behaviour are also the significant features of nanomaterials. Hence, nanoparticles can be used as a promising treatment regimen for the mitigation and cure of periodontal disorders.
Chlorhexidine loaded nanomaterials such as nanosponges and nanocapsules showed sustained and controlled release of the drug over a large surface area, responsive to different pH with the advantage of penetrating and infiltrating the gum tissue gingiva subgingivally. These formulations have been found to provide better patient compliance over the conventional dosage forms in reducing the chances of periodontal diseases by destroying and restricting the formation of dental plaques, microbial biofilm and tartars by several virulent microbial species, the major factors behind the onset of periodontitis and gingivitis [98]. Nanofiber membranes and electrospun fibers will open new avenues towards periodontal regenerative medicinal policies [99].
i) 3D-Printing
3D printing, an advanced modern technology, is aimed at producing and building materials based upon successive layer formation by an additive manufacturing approach. Exact replica of each product is obtained by a 3D printer, which uses the stored information from the CAD software, measuring the multiple crosssections. Fabrication of stone models, development of custom impression trays and dental prosthesis are some of the 3D-printed techniques which have wide applications in dentistry. Investigation of tissue scaffolding in bone grafting procedures can be done with the help of 3D printer. The advantages are many starting thorough preoperative planning, improved accuracy to fit of prosthesis with lesser time consumption. 3D wax printing and fused deposition modelling techniques can be used as an essential functional tool for facilitating the morphogenesis of the periodontal tissue complex.
j) Development of some novel dental formulations
Dental disorders such as toothache, dental caries, gingivitis and oral malodour (Halitosis) or foul smell from the mouth are some of the serious problems amongst people of all age groups globally. In the oral cavity, bacterial invasions and release of bacterial toxins lead to bleeding of the gum (gingivitis) and when it spreads to the deeper tissues, the entire periodontal region is affected. Bacterial growth due to accumulation of food particles in the holes or cavities formed in the enamel and dentin part of a tooth, damage the dental pulp (inner living tissue of the tooth) and damage the nerve cells of the pulp. This leads to tremendous pain in the oral region along with foul smell. Oral antibiotics and painkillers are associated with various side effects such as hyperacidity, gastric irritation, slow onset of action and various others. In addition, most of the dental or oral formulations are liquid type solutions which get washed away readily with watery saliva [100].
In a different study, Manasadeepa et al. [102] reported about formulating and characterizing an innovative dental patch with efficient mucoadhesive properties and pressure sensitiveness. Polymers with confirmed biocompatibility, biodegradability and non-toxicity were chosen for developing this type of formulation. Combination of an antibiotic agent and an analgesic cargo was used to develop a number of experimental dental patches. Different formulations were developed, characterized and compared in context of various evaluating factors with the aim of providing sustained and controlled release of therapeutic agents, prolonged duration of action, more residence time in the oral region, better bioavailability, reduced drug related toxicities, better patient compliance for the treatment and management of periodontal diseases and improvements in maintenance of proper oral hygiene.
Figure 3 pictorially demonstrates the prospects of dental modularizes of periodontal diseases.
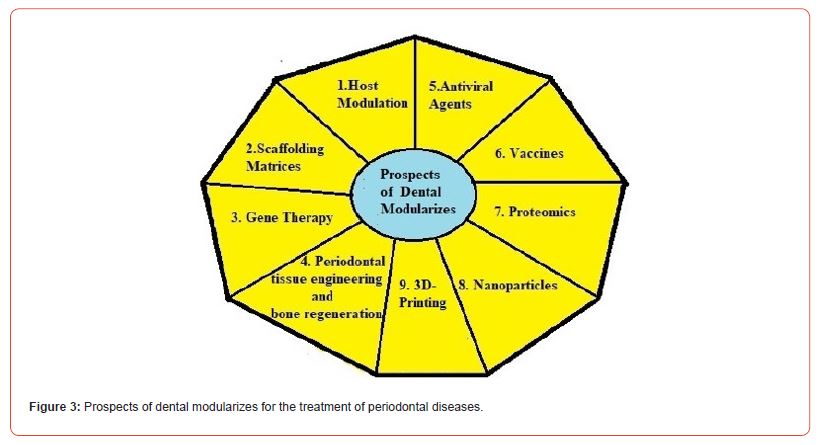
Conclusion
Thus, we can conclude, that the current state of periodontal disease treatment strategies shows diversified approaches that include non-surgical and surgical procedures, as well as developing therapeutics based on precision medicine, microbiome research, and immunomodulation. While these treatments have shown tremendous promise in terms of improving periodontal results, some lacunae persist such as the need for more standardized treatment procedures, more access to sophisticated therapies, and improved patient education. Future research in periodontal care will focus on personalized treatment strategies based on genetic and microbiological profiles, the development of minimally invasive treatments, and the ongoing integration of technology. Research and development of specific biomarkers for differentiating between different types of periodontitis is necessary in the context of both present and future disease perspectives. Proper investigations are required for the prevention of the disease in terms of community and population.
Although the prevailing treatment modalities have significant advantages such as restricting the progression of the disease and reinstallation of some lost periodontal features that support our dental and oral structures, extensive research demands for the development of novel treatment modalities with costeffectiveness, availability, less invasiveness based on our ideas about tissue regeneration and repairment. Multicentered randomized controlled clinical trials are necessary to figure out if these treatment modalities have the chance of giving rise to certain systemic diseases or not. As we gain a better understanding of the complex relationship between dental and systemic health, a more holistic and proactive approach to periodontal treatment is on the horizon, potentially reshaping the future of oral care, and overall well-being, and opening a new era in periodontology with better patient compliance.
Acknowledgement
We acknowledge Dr. V. Ravichandran Center of Advanced Research in Pharmaceutical Sciences, in Jadavpur University, to provide technical and literature support.
Conflict of Interest
Authors report no conflict of interest.
References
- Pihlstrom BL, Michalowicz BS, Johnson NW (2005) Periodontal diseases. Lancet 366(9499): 1809-1820.
- Loe H, Anerud A, Boysen H (1986) Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol 13: 431-445.
- Loe H, Anerud A, Boysen H (1978) The natural history of periodontal disease in man. The rate of periodontal destruction before 40 years of age. J Periodontol 49: 607-620.
- Albandar JM, Rams TE (2002) Global epidemiology of periodontal diseases. Periodontol 2000 Copenhagenm Denmark Munksgaard Blackwells 29.
- Kinane DF, Stathopoulou PG, Papapanou PN (2017) Periodontal diseases. Nat Rev Dis Primers 3: 17038.
- Lalla E, Papapanou PN (2011) Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol 7(12): 738-748.
- Stewart R, West M (2016) Increasing evidence for an association between periodontitis and cardiovascular disease. Circulation 133(6): 549-551.
- Gomes Filho IS, Cruz SSD, Trindade SC, Passos Soares JS, Carvalho Filho PC, et al. (2020) Periodontitis and respiratory diseases: a systematic review with meta-analysis. Oral Dis 26(2): 439-446.
- Ide M, Harris M, Stevens A, Sussams R, Hopkins V, et al. (2016) Periodontitis and cognitive decline in Alzheimer’s disease. PLoS One 11(3): e0151081.
- Axelsson P, Lindhe J (1981) The significance of maintenance care in the treatment of periodontal disease. J Clin Periodontol 8: 281-294.
- Renvert S, Persson GR (2004) Supportive periodontal therapy. Periodontol 2000 36: 179-195.
- Darveau R P (2010) Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 8: 481-490.
- Lourenco TG (2014) Microbial signature profiles of periodontally healthy and diseased patients. J Clin Periodontol 41: 1027-1036.
- Perez Chaparro PJ (2014) Newly identified pathogens associated with periodontitis: a systematic review. J Dent Res 93: 846-858.
- Perez Chaparro P J (2016) The current weight of evidence of the microbiologic profile associated with peri-implantitis: a systematic review. J Periodontol 87: 1295-1304.
- Feres M, Teles F, Teles R, Figueiredo LC, Faveri M (2016) The subgingival periodontal microbiota of the aging mouth. Periodontol 2000 72: 30-53.
- Haubek D (2008) Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet 371: 237-242.
- Amaliya A (2015) Java project on periodontal diseases: periodontal bone loss in relation to environmental and systemic conditions. J Clin Periodontol 42: 325-332.
- Mombelli A, Casagni F, Madianos PN (2002) Can presence or absence of periodontal pathogens distinguish between subjects with chronic and aggressive periodontitis? A systematic review. J Clin Periodontol 29: 10-21.
- Pillet S (2016) Cytomegalovirus and ulcerative colitis: place of antiviral therapy. World J Gastroenterol 22: 2030.
- Kinane DF, Demuth DR, Gorr SU, Hajishengallis GN, Martin MH (2007) Human variability in innate immunity. Periodontol 2000 45: 14-34.
- Hajishengallis G, Lamont R J (2012) Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol 27: 409-419.
- Kinane DF, Hajishengallis G (2009) Polymicrobial infections, biofilms, and beyond. J Clin Periodontol 36: 404-405.
- Benakanakere M, Kinane DF (2012) Innate cellular responses to the periodontal biofilm. Front Oral Biol 15: 41-55.
- Graves D (2008) Cytokines that promote periodontal tissue destruction. J Periodontol 79: 1585-1591.
- Gemmell E, Seymour G J (2004) Immunoregulatory control of Th1/Th2 cytokine profiles in periodontal disease. Periodontol 2000 35: 21-41.
- Aranha AM (2012) Evidence supporting a protective role for Th9 and Th22 cytokines in human and experimental periapical lesions. J Endod 39: 83-87.
- Shapira L, Wilensky A, Kinane DF (2005) Effect of genetic variability on the inflammatory response to periodontal infection. J Clin Periodontol 32: 72-86.
- Baylin SB (2005) DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol 2: S4-S11.
- Bermejo Fenoll A, Sanchez Perez A (2004) Necrotising periodontal diseases. Med Oral Patol Oral Cir Bucal 9: 114-119.
- Kulkarni C, Kinane DF (2014) Host response in aggressive periodontitis. Periodontol 2000 65: 79-91.
- Löe H, Theilade E, Jensen SB (1965) Experimental Gingivitis in Man. J Periodontology 36(3): 177-187.
- Van der Weijden GA, Timmerman MF, Nijboer A, Lie MA, Van der Velden U (1993) A comparative study of electric toothbrushes for the effectiveness of plaque removal in relation to toothbrushing duration: Timerstudy. J Clin Periodontol 20(7): 476-481.
- Ridgeway EE (2000) Periodontal disease: diagnosis and management. J Am Acad Nurse Pract 12(3): 79-84.
- Pietruska M, Paniczko A, Waszkiel D, Pietruski J, Bernaczyk A (2006) Efficacy of local treatment with chlorhexidine gluconate drugs on the clinical status of periodontium in chronic periodontitis patients. Adv Med Sci 51 Suppl 1:162-165.
- Kumar AJ, Ramesh Reddy BV, Chava VK (2014) Effect of chlorhexidine chip in the treatment of chronic periodontitis. J Nat Sci Biol Med 5(2): 268-272.
- Lu HK, Chei CJ (2005) Efficacy of subgingivally applied minocycline in the treatment of chronic periodontitis. J Periodontal Res 40(1): 20-27.
- Blair FM, Chapple IL (2014) Prescribing for periodontal disease. Prim Dent J 3(4): 38-43.
- Leszczyńska A, Buczko P, Buczko W, Pietruska M (2011) Periodontal pharmacotherapy - an updated review. Adv Med Sci 56(2): 123-131.
- Barca E, Cifcibasi E, Cintan S (2015) Adjunctive use of antibiotics in periodontal therapy. J Istanb Univ Fac Dent 49(3): 55-62.
- Ohlrich EJ, Cullinan MP, Seymour GJ (2009) The immunopathogenesis of periodontal disease. Aust Dent J 54: S2-S10.
- Golub LM, Suomalainen K, Sorsa T (1992) Host modulation with tetracyclines and their chemically modified analogues. Curr Opin Dent 2: 80-90.
- Preshaw PM, Hefti AF, Novak MJ (2004) Subantimicrobial dose doxycycline enhances the efficacy of scaling and root planing in chronic periodontitis: a multicenter trial. J Periodontol 75: 1068-1076.
- Caton JG, Ciancio SG, Blieden TM (2000) Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol 71: 521-532.
- Offenbacher S, Odle BM, van Dyke TE (1986) The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontol Res 21: 101-112.
- Nyman S, Schroeder HE, Lindhe J (1979) Suppression of inflammation and bone resorption by indomethacin during experimental periodontitis in dogs. J Periodontol 50: 450-461.
- Williams RC, Jeffcoat MK, Kaplan ML, Goldhaber P, Johnson HG, et al. (1985) Flurbiprophen: a potent inhibitor of alveolar bone resorption in beagles. Science 227: 640-642.
- Offenbacher S, Braswell LD, Loos AS (1987) Effects of flurbiprophen on the progression of periodontitis in Macaca mulatto. J Periodontol Res 22: 473-481.
- Giannobile WV (2008) Host-response therapeutics for periodontal diseases. J Periodontol 79: 1592-1600.
- Reddy MS, Geyrs NC, Gunsolley JC (2003) Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents: A systematic review. Ann Periodontol 8: 12-37.
- Graziani F, Karapetsa D, Mardas N, Leow N, Donos N (2018) Surgical treatment of the residual periodontal pocket. Periodontology 2000 76(1): 150-163.
- Ferraiolo D (2016) Predicting periodontitis progression? Evid Based Dent 17: 19-20.
- Garrett S, Bogle G (1994) Periodontal regeneration with bone grafts. Curr Opin Periodontol 168-177.
- Kim David M, Neiva R (2015) Periodontal soft tissue non-root coverage procedures: a systemic review from the AAP regeneration workshop. J Periodontol 86(25): 556-572.
- Evian CI, al-Maseeh J, Symeonides E (2003) Soft tissue augmentation for implant dentistry. Compend Contin Educ Dent 24(3): 195-198.
- Ishikawa I, Aoki A, Takasaki AA, Mizutani K, Sasaki KM, Izumi Y (2009) Application of lasers in periodontics: true innovation or myth? Periodontology 2000 50(1): 90-126.
- Bader HI (2000) Use of lasers in periodontics. Dental Clinics of North America 44(4): 779-791.
- Coleton S (2004) Lasers in surgical periodontics and oral medicine. Dent Clin North Am 48(4): 937-962.
- Kumar PS (2021) Microbial dysbiosis: The root cause of periodontal disease. J Periodontol 92(8): 1079-1087.
- Gillam DG, Turner W (2014) Antibiotics in the Treatment of Periodontal Disease: A Guide for the General Dental Practitioner. Prim Dent J 3(3): 43-47.
- Lin NH, Gronthos S, Bartol PM (2008) Stem cells and periodontal regeneration. Australian dental journal 53(2): 108-121.
- Mitrano TI, Grob MS, Carrion F, Nova Lamperti E, Luz PA, et al. (2010) Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol 81(6) :917-925.
- Chen FM, Sun HH, Lu H, Yu Q (2012) Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 33(27): 6320-6344.
- Wang M, Xie J, Wang C, Zhong D, Xie L, et al. (2020) Immunomodulatory Properties of Stem Cells in Periodontitis: Current Status and Future Prospective. Stem Cells Int: 9836518.
- Malcangi G, Patano A, Guglielmo M, Sardano R, Palmieri G, et al. (2023) Precision Medicine in Oral Health and Diseases: A Systematic Review. J Pers Med 13(5): 725.
- Kikuchi T, Hayashi JI, Mitani A (2022) Next-Generation Examination, Diagnosis, and Personalized Medicine in Periodontal Disease. J Pers Med 12(10): 1743.
- Gritz ER, Vidrine DJ, Fingeret MC (2007) Smoking cessation a critical component of medical management in chronic disease populations. Am J Prev Med 2007 33(6 Suppl): S414-22.
- Zhang Y, He J, He B, Huang R, Li M (2019) Effect of tobacco on periodontal disease and oral cancer. Tob Induc Dis 17: 40.
- da Costa FD, Prashant GM, Sushanth VH, Imranulla M, Prabhu A, et al. (2019) Assessment of knowledge, attitude and practices of diet and nutrition on oral health among dental students. J Global Oral Health 2(1): 29-35.
- Tonetti MS, Jepsen S, Jin L, Otomo Corgel J (2017) Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J Clin Periodontol 44(5): 456-462.
- Păunică I, Giurgiu M, Dumitriu AS, Păunică S, Pantea Stoian AM, et al. (2023) The Bidirectional Relationship between Periodontal Disease and Diabetes Mellitus: A Review. Diagnostics (Basel) 13(4): 681.
- Newton JT, Asimakopoulou K (2015) Managing oral hygiene as a risk factor for periodontal disease: a systematic review of psychological approaches to behaviour change for improved plaque control in periodontal management. J Clin Periodontol 42: S36-S46.
- Lakschevitz F, Aboodi G, Tenenbaum H, Glogauer M (2011) Diabetes and periodontal diseases: interplay and links. Curr Diabetes Rev 7(6): 433-439.
- Zhou M, Dong J, Zha L, Liao Y (2021) Causal Association between Periodontal Diseases and Cardiovascular Diseases. Genes (Basel) 13(1): 13.
- Tsirigotis P, Chondropoulos S, Gkirkas K, Meletiadis J, Dimopoulou I (2016) Balanced control of both hyper and hypo-inflammatory phases as a new treatment paradigm in sepsis. J Thorac Dis 8: E312-E316.
- Elavarasu S, Sekar S, Murugan T (2012) Host modulation by therapeutic agents. J Pharm Bioallied Sci 4: S256-S259.
- Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, et al. (2018) Periodontitis: Consensus report of workgroup 2 of the 2017 WorldWorkshop on the classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol 45: S162-S170.
- Van Dyke TE, Hasturk H, Kantarci A, Freire MO, Nguyen D, et al. (2015) Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res 94: 148-156.
- El-Sharkawy H, Aboelsaad N, Eliwa M, Darweesh M, Alshahat M, et al. (2010) Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 Fatty acids and low-dose aspirin. J Periodontol 81: 1635-1643.
- Hasturk H, Kantarci A, Goguet Surmenian E, Blackwood A, Andry C, et al. (2007) Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol 179: 7021-7029.
- Hubbell J (2008) Controlled release strategies in tissue engineering. In van Blitterswijk, C editor Tissue Engineering Burlington VT Academic Press: 455-482.
- Lutolf MP, Weber FE, Schmoekel HG (2003) Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol 21: 513-518.
- Sugano M, Egishi Y, Endo Takahashi Y, Hamano N, Usui M, et al. (2014) Gene delivery to periodontal tissue using Bubble liposomes and ultrasound. J Periodontal Res 49: 398-404.
- Peng L, Cheng X, Zhuo R, Lan J, Wang Y, et al. (2009) Novel gene-activated matrix with embedded chitosan/plasmid DNA nanoparticles encoding PDGF for periodontal tissue engineering. J Biomed Mater Res A 90: 564-576.
- Edwards PC, Mason JM (2006) Gene-enhanced tissue engineering for dental hard tissue regeneration: overview and practical considerations. Head Face Med 2 (12).
- Chen Z, Guo Z, Lin H, Tian Y, Zhang P, et al. (2021) The feasibility of phage therapy for periodontitis. Future Microbiol 16: 649-656.
- Matthew Galli, Yao Yao, William V Giannobile, Hom Lay Wang (2021) Current and future trends in periodontal tissue engineering and bone regeneration. Plast Aesthet Res 8(3): 176.
- Balaji TM, Varadarajan S, Sujatha G, Muruganandhan J, Shanmugapriya R, et al. (2021) Necrotizing periodontal diseases in human immunodeficiency virus-infected patients receiving highly active antiretroviral therapy: A review. Dis Mon 67: 101168.
- Cappuyns I, Gugerli P, Mombelli A (2005) Viruses in periodontal disease: A review. Oral Dis 11: 219-229.
- Takahashi Y, Kumada H, Hamad N, Haishima Y, Ozono S, Isaka M, Yasuda Y, Tochikubo K, Umemoto T (2007) Induction of immune responses and prevention of alveolar bone loss by intranasal administration of mice with Porphyromonas gingivalis fimbriae and recombinant cholera toxin B subunit. Oral Microbiol Immunol 22: 374-380.
- Herath TDK, Darveau RP, Seneviratne CJ, Wang CY, Wang Y, et al. (2016) Heterogeneous Porphyromonas gingivalis LPS modulates immuno-inflammatory response, antioxidant defense and cytoskeletal dynamics in human gingival fibroblasts. Sci Rep 6: 29829.
- Coutinho A, Reddy N, Chatterjee A, Khan MI (2021) The Role of Visfatin (Adipocytokine) Biomarker in Oral Health and Diseases among Nonobese Indian Population: A Proteomic Assay. Glob Med Genet 8: 104-108.
- Choi YJ, Heo SH, Lee JM, Cho JY (2011) Identification of azurocidin as a potential periodontitis biomarker by a proteomic analysis of gingival crevicular fluid. Proteome Sci 9 (42).
- Hocevar K, Vizovišek M, Wong A, Kozieł J, Fonovic M, et al. (2020) Proteolysis of Gingival Keratinocyte Cell Surface Proteins by Gingipains Secreted From Porphyromonas gingivalis—Proteomic Insights Into Mechanisms Behind Tissue Damage in the Diseased Gingiva. Front Microbiol 11: 722.
- Liu S, Wang Y, Ma B, Shao J, Liu H, et al. (2021) Gingipain-Responsive Thermosensitive Hydrogel Loaded with SDF-1 Facilitates In Situ Periodontal Tissue Regeneration. ACS Appl Mater Interfaces 13: 36880-36893.
- Johnson A, Kong F, Miao S, Lin H TV, Thomas S, Huang YC, Kong ZL (2020) Therapeutic effects of antibiotics loaded cellulose nanofiber and κ- carrageenan oligosaccharide composite hydrogels for periodontitis treatment. Sci Rep10: 18037.
- Baranov N, Popa M, Atanase LI, Ichim DL (2021) Polysaccharide-Based Drug Delivery Systems for the Treatment of Periodontitis. Molecules 26: 2735.
- Vidal Romero G, Zambrano Zaragoza ML, Martínez Acevedo L, Leyva Gómez G, Mendoza Elvira SE, et al.(2019) Design and evaluation of pH-dependent nanosystems based on cellulose acetate phthalate, nanoparticles loaded with chlorhexidine for periodontal treatment. Pharmaceutics 11: 604.
- Wang Y, Jiang Y, Zhang Y, Wen S, Wang Y, et al. (2019) Dual functional electrospun core-shell nanofibers for anti-infective guided bone regeneration membranes. Mater Sci Eng C Mater Biol Appl 98.
- Ghosh S, Roy G, Mukherjee B (2009) Dental Mold: A Novel Formulation to Treat Common Dental Disorders. AAPS PharmSciTech 10(2).
- Mukherjee B, Roy G, Ghosh S (2009) Development of Denticap, a Matrix Based Sustained Release Formulation for Treatment of Toothache, Dental Infection and Other Gum Problem. Curr Drug Deliv 2009 6: 199-207.
- Manasadeepa R, Paul P, Mukherjee B (2013) Pressure-sensitive mucoadhesive polymer-based dental patches to treat periodontal diseases: an in vitro study. Drug Deliv 20(6): 258-267.
-
Sandipan Mallick, Mrinmoy Barman, Sefali Halder Hota and Biswajit Mukherjee*. Present and Future Treatment Modalities for the Mitigation and Cure of Periodontal Diseases. On J Dent & Oral Health. 7(2): 2023. OJDOH.MS.ID.000660.
-
Periodontal diseases, Dental devices, Dental caries, Toothache, Dental plaques, Gum tissue, Mouth, Periodontitis, Dental implants, Loosened teeth
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






