 Research Article
Research Article
Efficacy of some Antibiotics against Streptococcus Mutans Associated with Tooth decay in Children and their Mothers
Ibrahim Zaid Al-Shami1, Mohsen Ali Al-Hamzi1, Hassan A Al-Shamahy2* and Arij Lutf Abdulrhman Abdul Majeed2
1Department of Conservative Dentistry and Oral Health, Faculty of Dentistry, Sana’a University, Republic of Yemen, Yemen
2Medical Microbiology and Clinical Immunology, Faculty of Medicine and Health Sciences, Sana’a University, Republic of Yemen, Yemen
Prof. Hassan A Al-Shamahy, Faculty of Medicine and Heath Sciences, Sana’a University, P.O. Box 775 Sana’a, Yemen.
Received Date: April 13, 2019; Published Date: May 16, 2019
Abstract
Background:Dental caries is recognized as one of the most infectious diseases worldwide and Mutans streptococci (MS) have been commonly associated as major cariogenic bacteria.
Objectives: The objective of this study was to identify and determine the antibiogram profile of Streptococcus mutans associated with tooth mutans associated with tooth decay in children and their mothers.
Methods: The dental plaque samples were collected from caries active subjects children group (aged 2-5 years) and mother group (aged 35-44 years) at dental clinics of Sana’a University in Sana’a city, Yemen. S. mutans identified by standard bacteriological methods and 87 clinical isolates S. mutans form mothers and 87 clinical isolates S. mutans from children were tested for antibiogram profile. Antibiogram profiling was performed to determine the susceptibility of 6 β-Lactam antibiotics (penicillin, ampicillin, cefotaxime, amoxicillin, cefazolin and methicillin) and 4 non β-Lactam antibiotics (erythromycin, lincomycin, clindamycin and vancomycin) by disc diffusion method.
Results: Ampicillin, cefotaxime cefazolin, methicillin and clindamycin were the most effective antibiotics against S. mutans isolates and resistance rate for them do not exceed 2.3%. The highest resistance rates were against erythromycin (24.1%), lincomycin (28.7%) followed by penicillin (14.9% in children S. mutans isolates) and amoxicillin (14.9% in mother S. mutans isolates).
Conclusion: The study demonstrates significant levels of penicillin, erythromycin, amoxicillin, clindamycin and lincomycin-resistance in S. mutans clinical isolates in dental patients. Further study is required to know the minimum inhibitory concentration of β-Lactam and non β-Lactam antibiotics. These results also, call for improved the assessment of antibiotic susceptibility testing during prophylaxis. The alternative of antibiotic such as herbal extract is most likely preferable for the coming years to avoid the upcoming bacterial resistance to the antibiotics.
Keywords:Antibiotic susceptibility testing; Streptococcus mutans associated with tooth mutans; Dental caries; Children; Mothers
Introduction
Dental caries is recognized as one of the most infectious diseases worldwide [1,2]. Mutans streptococci (MS) have been commonly associated as major cariogenic bacteria. S. mutans is present in oral flora and has been demonstrated to be a causative specialist for dental caries because of its capacity to metabolize fermentable carbohydrate into organic acids. These acids can cause a fall in pH, which can lead to an increase of enamel solubility that is dental caries [3]. Expanding resistance of bacterial pathogens to regularly utilize antibiotics has turned into general human concern. The spread of antibiotic resistance is causing fatalities, as well as a high financial inconvenience. In low economic nations as Yemen, antibiotic resistance is more prevalent than in the developed countries [4].
S. mutans is also included as a causative agent of endocarditis. Information about the antibiogram profile of S. mutans is of significance for prescribing the appropriate treatment in the case of endocarditis [5]. One-hour prior dental procedure, the American Heart Association suggests antimicrobial prophylaxis for high-risk cardiovascular patients, such as amoxicillin (2g) as first choice and clindamycin (600mg) as a second choice [6]. Production of β-lactamase is, however, unusual for most of streptococci, where resistance is happening by slightly altered of penicillin binding proteins [7-9]. In 2012 investigators have accounted a significant level of penicillin resistance 13.4% of 550 oral streptococcal clinical isolates, out of 50 isolates of S. mutans 14% were resistant to penicillin [10]. Consistent with the study performed in 2014, 38 isolates of S. mutans showed a complete resistance to penicillin and ampicillin [11]. Bacterial resistance to antibiotics such as penicillin and other β-lactam is a health issue in numerous parts of the world. Thus, the objective of this study was to identify and determine the antibiogram profile of Streptococcus mutans associated with tooth decay in children and their mothers.
Materials and Methods
The present study was conducted in the Department of Conservative Dentistry and Oral Health, Faculty of Dentistry, Sana’a University, Republic of Yemen. The study protocol was approved by the ethics committee of Sana′a University. A written informed consent was obtained from the selected participants. An eightyseven plaque dental samples were collected from caries active mothers and 87 plaque dental samples were collected from their caries active children.
Microbiological procedure
The antibiotic susceptibility profile was determined by disc diffusion method. The inoculums were adjusted to match the turbidity of 0.5 McFarland standards, and was swabbed on Brian heart infusion agar and allowed to dry for 10min [12]. The antibiotics employed in this study were: penicillin-G (P) 10 units, ampicillin (AMP) 10μg, cefotaxime (CTX) 30μg, erythromycin (E) 15μg, cefazolin (CZ) 30μg, methicillin (MET) 5μg, lincomycin (L) 2μg, clindamycin (CC) 2μg and vancomycin V (30μg) (Oxide, USA). Inhibition zone was measured after 24h of anaerobically incubation at 37 °C. The experiments of each antibiotic were performed in triplicate. The results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) methodology [13].
Results
Table 1 presented the pattern of antibiotic sensitivity ofS. mutans isolates from carries mother patients and caries children patients. Ampicillin, cefotaxime cefazolin, methicillin and clindamycin were the most effective antibiotics against S. mutans isolates and resistance rate do not exceed 2.3%. The isolatedS. mutans shows a high resistance rate against erythromycin (children isolates= 24.1%), and against lincomycin (mother isolates 28.7%). The resistance rate against penicillin was 14.9% in childrenS. mutans isolates and 8% inS. mutans mother isolates. The resistance rate against amoxicillin was 14.9% in motherS. mutans isolates and 12.6% inS. mutans children isolates.
Discussion
Most of the antibiotics employed in this study are commonly prescribed by dentists [2,14]. The number of resistant of oral mutans streptococci is greater in people frequently exposed to antibiotics, although the resistant bacteria may also be found in healthy subjects who have not been recently treated with antibiotics [2]. β-lactam antibiotics are the most commonly prescribed chemo prophylactic agent’s in general dental practices. However, resistance to penicillin among oral streptococci is increasing [15]. The number of resistant oral streptococci is greater in people frequently exposed to antibiotics [16], although these bacteria may also be found in healthy subjects who have not been recently treated with an antimicrobial [17].
Bacterial resistance to antibiotics such as penicillin and other β-lactam is a health issue in numerous parts of the world. In our study we observed a significant level of penicillin resistance (14.9%) in children Strept mutans clinical isolates. The high prevalence of resistance to penicillin in Strept. mutans in our study is like that previously observed in South Africa and Spain in oral streptococcus viridans [18,19]. Several in-vitro studies have demonstrated the capability to transfer penicillin resistance determinants among related species [20]. These mechanisms, together with selective antibiotic pressure, may play an important role in the emergence and spread of penicillin resistance in oral streptococci.
Also, the significant level of penicillin resistance (14.9%) in Strept. mutans clinical isolates in our study is similar to Pasquantonio et al. [10] study that reported a significant level of penicillin resistance: 13.4% of 550 oral streptococcal clinical isolates, out of 50 isolates of S. mutans 14% were resistant to penicillin [10]. However, our result is lower than the rate of a study conducted in 2014 by Dhamodhar et al. [11] in which 38 isolates of S. mutans showed a complete resistance to penicillin and ampicillin. One-hour prior dental procedure, the American Heart Association suggests antimicrobial prophylaxis for high-risk cardiovascular patients, such as amoxicillin (2g) as first choice and clindamycin (600mg) as a second choice [6]. Production of β-lactamase is, however, unusual for most of streptococci, where resistance is happening by slightly altered of penicillin binding proteins [7-9].
However, in our study we observed a significant level of amoxicillin resistance [14.9%) in mother isolates and (12.6%) in children isolates of Strept. mutans; and 8% for clindimycin in children isolates. Thus, in this condition first choice should be go to third generation of cephalosporin’s and ampicillin in which resistance to cephalosporins and ampicillin is less than 2% (Table 1). The children isolate of S. mutans showed slightly more susceptibility against tested antibiotics employed in this study than the mother isolates of S. mutans. This different might be due to long exposure of mothers to these antibiotics than their children. However, a significant level of erythromycin resistance (24.1%) in children isolates than mother isolates (10.3%).
Table 1: The antibiotic sensitivity test for S. mutans clinical isolates from mothers and their children.
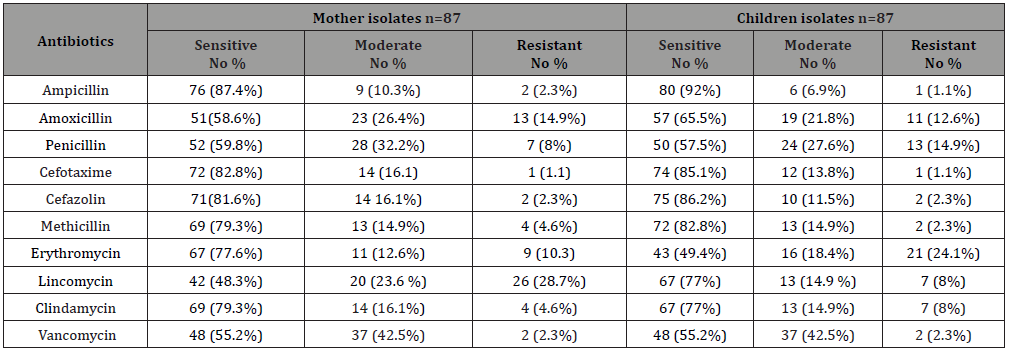
The high rate of erythromycin resistance in children isolates can be explained by selective antibiotic pressure, in which erythromycin is used more common in children than in adults; and this may play an important role in the emergence and spread of erythromycin resistance in children isolates. Ultimately, the resistant developed by S. mutans is obscure. Updated information on antibiotic susceptibility testing such as reported in the present study helps to notify pharmaceutical makers to design new strategies for effective prophylaxis against dental infections. This result also gives an ideal choice to the dentist to prescribe a suitable antibiotic in Yemen
Conclusion
Our study demonstrates significant levels of penicillin, erythromycin, amoxicillin, clindamycin and lincomycin-resistance in S. mutans clinical isolates in dental patients. Isolates were more susceptible to ampicillin, cefotaxime, and cefazolin than others tested antibiotics. Further study is required to know the minimum inhibitory concentration of β-Lactam and non β-Lactam antibiotics. These results also, call for improved the assessment of antibiotic susceptibility testing during prophylaxis. The alternative of antibiotic such as herbal extract is most likely preferable for the coming years to avoid the upcoming bacterial resistance to the antibiotics. In addition, the rise in the rate of antibiotic resistance in S. mutans clinical isolates suggested taking extra precaution while prescribing antibiotics will maintain the bacteria with less
Acknowledgement
The authors would like to acknowledge Sana’a University, Sana’a, Yemen which supported this work.
Conflict of Interest
No conflict of interest associated with this work.
References
- Okada T, Takada K, Fujita K, Ikemi T, Osgood RC, et al. (2011) Differentiation of banding patterns between Streptococcus mutans and Streptococcus sobrinus isolates in rep-PCR using ERIC primer. J Oral Microbiol 3: 7190.
- Salman HA, Senthikumar R (2017) Identification and Antibiogram Profile of Streptococcus mutans and Streptococcus sobrinus from Dental Caries Subjects. Contemp Clin Dent 5(06): 054-057.
- Hui SHH, Ariffin Z, Alam MK (1994) In Vitro Study of Antibacterial Properties of Endodontic Sealers and Medications towards Streptococcus mutans and Enterococcus feacalis. International Medical Journal 20(4): 493-495.
- Kapi A (2014) The evolving threat of antimicrobial resistance: Options for action. Indian J Med Res 139(1): 182.
- DeMoor CE, DeStoppelaar JD, Van Houte J (1972) The occurrence of mutans and S. sanguis in the blood of endocarditis patients. Caries Res 6:73.
- Dajani AS, Taubert KA, Wilson W (1997) Prevention of bacterial endocarditis. Recommendations by the American Heart Association. J Am Dent Assoc 96: 358-366.
- Chambers HF (1999) Penicillin-binding protein-mediated resistance in pneumococci and staphylococci. J Infect Dis 179: S353-359.
- Cvitkovitch DG (2001) Genetic competence and transformation in oral streptococci. Crit Rev Oral Biol Med 12: 217-243.
- Hakenbeck R (1998) Mosaic genes and their role in penicillin resistant Streptococcus pneumoniae. Electrophoresis 19: 597–601.
- Pasquantonio G, Condo S, Cerroni L, Bikiqu L, Nicoletti M, et al. (2012) Antibacterial activity of various antibiotics against oral streptococci isolated in the oral cavity. Int J Immunopathol Pharmacol 25(3): 805-809.
- Dhamodhar P, Sreenivasa Murthy, Channarayappa, Shanthakumar SS, Indiresha HN (2014) Prevalence, characterization and heterogeneity studies on Streptococcus mutans isolated from Bangalore urban population. Int J Pharm Bio Sci 5(3): 122-128.
- Jebashree HS, Kingsley SJ, Sathish ES, Devapriya D (2011) Antimicrobial activity of few medicinal plants against clinically isolated human cariogenic pathogens: An in vitro study. ISRN Dent 11: 67-72.
- CLSI (2012) Performance Standards for Antimicrobial Disc Susceptibility Tests. (11th), Approved standard M02-A11– Publication of Clinical and Laboratory Standards Institute [CLSI), USA, 32.
- Sweeney LC, Dave J, Chambers PA, Heritage J (2004) Antibiotic resistance in general dental practice-a cause for concern. J Antimicrob Chemother 53:567-576.
- (2010) European Committee on Antimicrobial Susceptibility Testing [EUCAST) Clinical breakpoints.
- Escribano E, Linares J, Alcaide F, Alonso T, Ayats J, et al. (1990) Increasing antimicrobial resistance among blood isolates of viridans” Streptococcus. Program and abstracts of the 30th Inter-science conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington DC, USA.
- Tozer RA, Boutflower S, Gillespie WA (1966) Antibiotics for the prevention of bacterial endocarditis during dental treatment. Lancet 1: 686-688.
- (1982) Working Party of the British Society for the Antimicrobial Chemotherapy The antibiotic prophylaxis of infective endocarditis. Lancet 2: 1323-1326.
- Potgieter E, Chalkley LJ (1991) Reciprocal transfer of penicillin resistance genes between Streptococcus pneumoniae, Streptococcus mitior and Streptococcus sanguis. J Antimicrob Chemother 28: 463-465.
- Sanchez R, Munoz P, Rodriguez-Creixems M, Pelaez T, Vasallo FJ, et al. (1994) Susceptibility pattern of Streptococcus viridans group isolated from blood cultures. Abstr. E5 program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington DC, USA.
-
Ibrahim Zaid Al-Shami, Mohsen Ali Al-Hamzi, Hassan A Al-Shamahy, Arij Lutf Abdulrhman Abdul Majeed. Efficacy of some Antibiotics against Streptococcus mutans Associated with Tooth decay in Children and their Mothers. On J Dent & Oral Health. 2(1): 2019. OJDOH.MS.ID.000530.
-
Molar impaction, Teeth predispose, Periodontal disease, Pericoronitis, Periodontitis, Cystic lesions, Neoplasm, Root resorption, Detrimental effects, Yemeni population, Eruption, Impaction state, Angulation, Yemeni adult, Mandibular
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






