 Research Article
Research Article
Postoperative Human Papilloma Virus Positivity Rate Due to Differences in Surgical Procedures in Patients with Cervical Intraepithelial Neoplasia
Yoko To, Naoki Abe, Saya Watanabe, Sachino Kira, Sotaro Hayashi, Shigeki Fujimoto, Miho Oda, Lifa Lee, Satoshi Nishiyama, Maki Goto, Fuyuki Eguchi and Hiroshi Tsujioka*
Department of Obstetrics and Gynecology, ASO Iisuka Hospital, Japan
Hroshi Tsujioka, Department of Obstetrics and Gynecology, ASO Iisuka Hospital 3-83 Yoshio-machi Iizuka-shi, Fukuoka, Japan.
Received Date:September 01, 2021; Published Date:October 11, 2021
Abstract
Objective: Cervical cancer is caused by human papilloma virus (HPV) infection, and cervical intraepithelial neoplasia (CIN) is a precancerous lesion. Common treatments for CIN are conization and laser vaporization. HPV testing is useful for predicting residual or recurrent disease after surgical treatment. We performed high-risk HPV (HR-HPV) genotyping postoperatively to examine the HPV positivity rate and determine whether this rate differed depending on the surgical procedure.
Methods: This study involved 42 patients with moderate to severe CIN (CIN2/3) who showed HR-HPV positivity before surgical treatment from May 2018 to July 2019. Of the 42 patients, 24 underwent conization and 18 underwent laser vaporization. HR-HPV genotyping was performed 6 months later, and cytology was carried out every 3 to 6 months postoperatively.
Results: Thirty-nine of the 42 patients (conization, n = 22; laser vaporization, n = 17) underwent HR-HPV genotyping 6 months after the surgery and were analyzed. Eight (20.5%) of these 39 patients were HR-HPV positive. There were no cases of HR-HPV negativity with abnormal cytology. Although there was no significant difference, analysis of the postoperative rate of HR-HPV positivity by surgical procedure (conization, n = 2; laser vaporization, n = 6) showed a higher positivity rate in the laser vaporization group (p = 0.059).
Conclusion: The postoperative HR-HPV positivity rate was about 20%. Patients who underwent laser vaporization had a higher HR-HPV positivity rate than those who underwent conization.
Keywords:HR-HPV; CIN; Conization; Laser vaporization
Introduction
The International Agency for Research on Cancer (IARC) reported that cervical cancer is the fourth most common cancer in women. Approximately 570,000 cases of cervical cancer and 311,000 deaths from this disease occurred in 2018 [1]. Chronic infection with certain types of human papillomavirus (HPV) is the main cause of cervical cancer and cervical intraepithelial neoplasia (CIN). More than 100 different HPV types have been described, and they can be further subdivided into low-risk and high-risk types according to their oncogenic potential [2]. There are 13 high-risk HPV (HR-HPV) types, including HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 [3-5]. In Japan, the indications for treatment of precancerous lesions are moderate CIN (CIN2) and positivity for HPV type 16, 18, 31, 33, 35, 45, 52, or 58 or severe CIN (CIN3). Treatment involves conization or laser vaporization. The pathologic diagnosis can be confirmed after performing conization. A negative margin is useful for predicting residual lesions and recurrence [6]. Although the relative risk of persistent or recurrent lesions is almost 5 times higher after excisional treatment with positive margins compared with negative margins (RR = 4.8; 95% CI = 3.2–7.2), only 56% (95% CI, 49–66%) of persistent/recurrent precancer was predicted by positive margin status [7,8]. In contrast, residual lesions cannot be evaluated after performing laser vaporization. Therefore, follow-up is required for risk evaluation even after the operation. HPV testing and/or vaginal cytology are performed for this purpose. HPV testing is more sensitive than cytology to find CIN2 or worse lesions, but its specificity is lower. Moreover, high-risk HPV positivity alone is not an indication for treatment, but strict follow-up is necessary. Hence, we performed HPV genotyping postoperatively, examined the HR-HPV positivity rate, and determined whether this rate differed depending on the surgical procedure.
Materials and Methods
This prospective observational study was performed at Aso Iizuka Hospital in Japan. All procedures performed in this study were approved by the Ethics Committee of Aso Iizuka Hospital, and all patients provided written informed consent. The study involved 42 patients with CIN2/3 who were confirmed to be HRHPV positive before surgical treatment from May 2018 to July 2019. Of the 42 patients, 24 underwent conization and 18 underwent laser vaporization. The surgical procedure was chosen by each patient. Patients who chose laser vaporization had a desire to bear children and it was acceptable provided the squamocolumnar junction was visible. Cold knife conization and carbon dioxide laser vaporization were used for the treatments. HPV genotyping was performed 6 months later, and cytology was carried out every 3 to 6 months postoperatively. Histological examination was performed when an abnormality was found on cytologic examination. HPV genotyping was assayed by the MEBGEN™ HPV kit (Medical and Biological Laboratories Co., Ltd., Tokyo, Japan). Thirteen high-risk carcinogenic HPV DNA types were detected (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) using the polymerase chain reaction– reverse sequence specific oligonucleotide method. Cervical cytology was assayed by the liquid-based cytology method.
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria) [9]. More precisely, EZR is a modified version of R commander (version 1.6-3) designed to add statistical functions frequently used in biostatistics. The patients were analyzed according to their age, the presence of HR-HPV, and their cytological results. Differences between the groups were tested using the Wilcoxon rank sum test or Fisher’s exact test. A p value of <0.05 was considered statistically significant.
Results
A total of 42 patients (conization, n = 24; laser vaporization, n = 18) underwent surgery. Preoperative HPV genotyping showed that 16 patients were positive for types 16 and 52, respectively, and that 12 were positive for type 58 (Table 1). The resection margins were positive in four patients who underwent conization. Thirtynine of the 42 patients (conization, n = 22; laser vaporization, n = 17) underwent HPV genotyping 6 months after the surgery, and we analyzed these patients as shown in Figure 1. The median age (interquartile range) at the time of surgery was 43 years (range, 33– 50 years) in the conization group and 31 years (range, 28–36 years) in the laser vaporization group. Although there was no significant difference in the postoperative HPV positivity rate according to the surgical procedure (p = 0.059), the HPV positivity rates were higher among patients who underwent laser vaporization (n = 6 [35.3%]) than patients who underwent conization (n = 2 [9.1%]). Among the patients with postoperative HR-HPV positivity, seven (87.5%) of eight patients had the same genotype as before the surgery. When HPV tests were negative, abnormal cytology was not observed. One of the two patients with HR-HPV positivity who underwent conization and had abnormal cytology postoperatively had a positive resection margin and the same HR-HPV type as before the operation. Cytologic examination showed atypical squamous cells of undetermined significance (ASC-US) at 6 months postoperatively, and histologic examination showed no malignancy at 7 months postoperatively. At 2 years 8 months postoperatively, examination revealed CIN2. Among the patients who underwent laser vaporization, three showed abnormal cytology and two showed a high-grade squamous intraepithelial lesion (HSIL), and histologic examination revealed HSIL/CIN3. HPV genotyping showed positivity for type 16, which was the same as before the surgery. The other patient showed ASC-US, but histological examination revealed no malignancy. The HPV genotype was different from that before the surgery. No abnormal cytology was found 9 and 12 months after the operation. The other four patients with HPV positivity (conization, n = 1; laser vaporization, n = 3) had no abnormal cytology results (Table 2).
Table 1: Results of preoperative HPV genotyping (with superinfection).
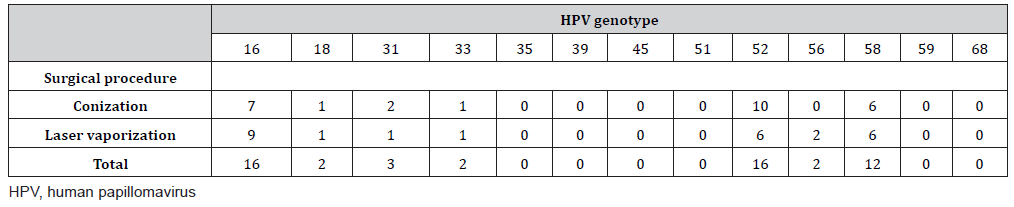
Table 2: Results of HPV testing and cytology by surgical procedure.
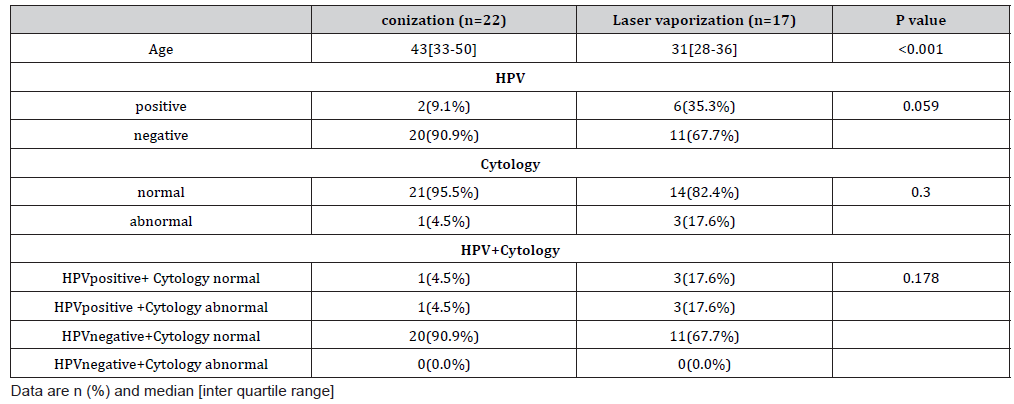
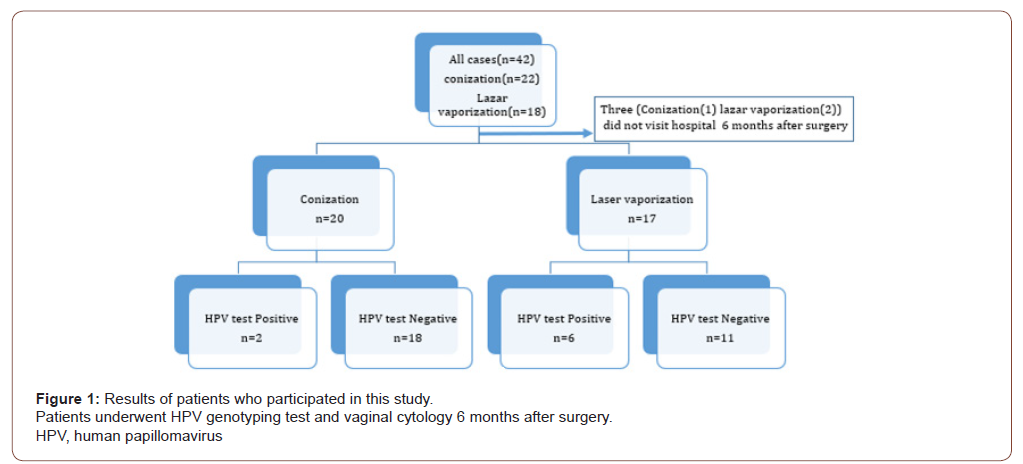
Discussion
According to the International Agency for Research on Cancer, cervical cancer is the fourth most common cancer in women. Approximately 570,000 cases of cervical cancer and 311,000 deaths from this disease occurred in 2018 [1]. Most HPV infections are transient and are not clinically problematic [10]. However, some patients are persistently infected and progress to CIN, carcinoma in situ, and invasive carcinoma. In Japan, in addition to HPV types 16 and 18, types 52 and 58 are also common in CIN2-3 [11]. The results of the present study were similar
(HPV16(n=16:38.1%)HPV52(n=16:38.1%) HPV58(n=12:28.5%))
Several studies have investigated the usefulness of HPV testing for residual and recurrent CIN lesions. Arbyn M, et al. [7], performed a meta-analysis showing that the sensitivity and specificity of detection of residual/recurrent lesions of CIN2 or higher were 91.0% and 84.4% in cases of positive margins, respectively and 91.0% and 83.4% in cases of high-risk HPV positivity, respectively. Additionally, the risk of residual and recurrent lesions was 3.7% for patients with negative margins and 0.8% for patients with highrisk HPV negativity. Kocken M, et al. [12], reported that cytology or HR-HPV testing was performed 6 months after surgery and that the sensitivity of detecting residual/recurrent lesions was 79% using cytology, 92% using HR-HPV testing, and 95% using both tests. Onuki M, et al. [13], reported that the detection sensitivity and specificity of cytology were 76% and 83%, those of HPV testing were 92% and 85%, and those of both tests were 93% and 76%, respectively. Additionally, for patients with positive surgical margins, HR-HPV testing provided remarkable risk discrimination between HR-HPV positivity and HR-HPV negativity (absolute risk of residual CIN2 or worse: 74.4% vs. 0.8%, respectively) [13]. The American Society of Colposcopy and Cervical Pathology recommends the performance of HPV testing 6 months after CIN2/3 treatment regardless of the margin status. If are negative, annual HPV or co testing is preferred until 3 consecutive negative tests have been obtained [8]. In the present study, four patients had positive margins. Only one patient was diagnosed with CIN2 by histological examination 2 years 8 months after treatment. In the other three patients, both postoperative cytology and HPV testing were negative. Although there was no significant difference (p = 0.059), the positivity rate of postoperative HPV testing was higher in the laser vaporization than conization group. The reason for choosing laser vaporization was a desire to bear children. Therefore, the chance of HR-HPV reinfection or another infection is higher than conization group. In addition, because it is not known whether residual lesions are present, patients with HR-HPV test positivity require more rigorous follow-up. Among patients with postoperative HPV positivity, 87.5% had the same genotype as before the surgery. There were two possible causes of this: persistent infection before the surgery or reinfection. No patients had both HPV negativity and abnormal vaginal cytology. If an HR-HPV test is negative, the risk of recurrence and residual disease is lower from this study and known reports. However, because the number of patients in this study was small, it is not known whether the positivity rate of postoperative HPV testing was higher in the laser vaporization than conization group. Further research is needed to determine.
Conclusion
The postoperative rate of HR-HPV positivity in this study was about 20%. The rate was higher in patients who underwent laser vaporization than conization.
Acknowledgement
This research was conducted with the assistance of the Aso Iizuka Hospital Clinical Research Grant. The authors thank all medical staff involved in this study. The authors also thank Angela Morben, DVM, ELS, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflict of Interest
The authors declare no competing interests.
Ethics Approval and Consent to Participate
All procedures performed in this study were approved by the Ethics Committee of Aso Iizuka Hospital. (the ethics approval number is 17045) All patients provided written informed consent.
Authors’ Contributions
YT and HT designed the research study. YT, SW, SK, MG performed the research and contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
References
- Arbyn M, Weiderpass E, Bruni L, Sanjosé S, Saraiya M, et al. (2018) Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 8(2): e191-e203.
- Baseman JG, Koutsky LA (2005) The epidemiology of human papillomavirus infections. J Clin Virol 32(Suppl 1): S16-S24.
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, et al. (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348(6): 518-527.
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, et al. (2010) Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11: 1048-1056.
- Bonde J, Rebolj M, Ejegod DM, Preisler S, Lynge E, et al. (2014) HPV prevalence and genotype distribution in a population-based split-sample study of well-screened women using CLART HPV2 human papillomavirus genotype microarray system. BMC Infect Dis 14: 413.
- Lu CH, Liu FS, Tseng JJ, Ho ES (2000) Predictive factors for residual disease in subsequent hysterectomy following conization for CIN Ⅲ. Gynecol Oncol 79(2): 284-288.
- Arbyn M, Redman CWE, Verdoodt F, Kyrgiou M, Tzafetas M, et al. (2017) Incomplete excision of cervical precancer as a predictor of treatment failure: a systematic review and meta-analysis. Lancet Oncol 18(12): 1665-1679.
- Rebecca BP, Richard SG, Philip EC, David C, Mark HE, et al. (2020) 2019 ASCCP Risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 24(2): 102-131.
- Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3): 452-458.
- Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, et al. (1999) Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis 180(5): 1415-1423.
- Tasuku M, Akira N, Kanae Sogawa, Riri Suzuki, Masae Saito, et al. (2016) Virological and cytological clearance in laser vaporization and conization for cervical intra-epithelial neoplasia grade 3. J Obstet Gynaecol Res 42(12): 1808-1813.
- Kocken M, Uijterwaal MH, de Vries ALM, Berkhof J, Ket JCF, et al. (2012) High-risk human papillomavirus testing versus cytology in predicting post-treatment disease in women treated for high-grade cervical disease: a systematic review and meta-analysis. Gynecol Oncol 125(2): 500-507.
- Onuki M, Matsumoto K, Sakurai M, Ochi H, Minaguchi T, et al. (2016) Posttreatment human papillomavirus testing for residual or recurrent high-grade cervical intraepithelial neoplasia: a pooled analysis. J Gynecol Oncol 27(1): e3.
-
Miho Oda, Lifa Lee, Satoshi Nishiyama, Maki Goto, Fuyuki Eguchi, Hiroshi Tsujioka. Postoperative Human Papilloma Virus Positivity Rate Due to Differences in Surgical Procedures in Patients with Cervical Intraepithelial Neoplasia. On J Complement & Alt Med. 7(1): 2021. OJCAM.MS.ID.000651.
-
HR-HPV; CIN; conization; laser vaporization; HPV positivity; Human Papilloma Virus; Surgical Procedures; Cervical Intraepithelial Neoplasia; Patients; Cytology
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






