 Research Article
Research Article
Longitudinal follow-up Study of the Intrarenal Hemodynamics and Pulmonary Function in Patients with T2DM
Lian Qun Jia1*, Xiao Lin Jiang1, Wei Chen1, He Tai1,2 and Jin Song Kuang3
1Key Laboratory of Ministry of Education for Traditional Chinese Medicine Visera-State Theory and Application, Liaoning University of Traditional Chinese Medicine, China
2Department of Endocrinology and Metabolic, Liaoning Provincial Corps Hospital of Chinese People’s Armed Police Forces, Shenyang, China
2Department of Endocrinology and Metabolic, Shenyang the Fourth Hospital of People, Shenyang, China
Lian Qun Jia, Liaoning University of Traditional Chinese Medicine, Huanggu District, Chongshan Road No. 79, Shenyang, Liaoning, 110847, China.
Received Date: February 25, 2020; Published Date: March 10, 2020
Keywords:
Abstract
Purpose: The main aim of this study is to long-range assess incidence of diabetic kidney disease (DKD) and pulmonary function in patients with type 2 diabetes mellitus (T2DM) during 2000 and 2015.
Methods: 60 subjects newly diagnosed with T2DM were divided into two groups according to their arterial resistivity index (RIs) (≤0.7 and >0.7). These patients underwent tests designed to assess the early changes in pulmonary function and intrarenal haemodynamics associated with diabetes, the results of which were compared to corresponding test results for the same group of patients between 2000 and 2015. The endpoints of the study were the between-group differences in the changes in the pulmonary functional parameters, the mean kidney arterial RI in the bilateral interlobular renal arteries, and the indicated renal functional parameters (BUN, Cr, AER, and UACR).
Results: The subjects with RIs ≤0.7 displayed significantly better pulmonary function in the year 2000 than subjects with RIs >0.7. Moreover, subjects in the former group displayed improvement and smaller decreases in their pulmonary functional parameters from 2000 to 2015 compared to subjects in the latter group. Additionally, subjects in the former group displayed improvement and smaller increases in their renal functional parameters, i.e., their BUN and Cr, and their AERs and UACRs from 2000 to 2015 compared to subjects in the latter group. The incidence of DKD in the group with RIs ≤0.7 (6/25, 24.00%) was significantly lower than that in the group with RIs >0.7 (23/45, 65.71%) (P<0.05).
Conclusion: The renal functional parameters, the right kidney RI may also serve as a predictor of diabetes-related changes in pulmonary function and renal injury in the future.
Keywords:Type 2 diabetes mellitus; Diabetic kidney disease; Pulmonary function; Intrarenal haemodynamics; Resistivity index
Abbrevations:Neurodegenerative disorders; Hypnosis; well-being; Embodiment; Relaxation; Complementary therapy
Introduction
The prevalence of T2DM is increasing worldwide, particularly in Asian countries [1]. Patients with T2DM develop abnormalities related to glucose and lipid metabolism, phenomena associated with multiorgan dysfunction. Moreover, T2DM has been identified as an independent risk factor for cardiovascular disease, as affected patients have a two-fold higher risk of developing cardiovascular disease than unaffected patients [2], and leads to the development of vascular diseases such as DN and DR [3], which are the leading causes of end-stage renal disease (ESRD) and acquired blindness, respectively [4]. New terminology describing kidney disease attributable to diabetes has been introduced in recent guidelines (National Kidney Foundation, 2007), which stipulate that the term ‘DN’ should be replaced by ‘DKD’, a major long-term microvascular complication of diabetes characterized by functional, structural and clinical abnormalities of the kidneys caused by diabetes that is highly prevalent among patients with the disease.
More than 3 decades ago, researchers established that patients with T2DM had less alveolar gas exchange capacity than healthy subjects [5]. However, hyperglycaemia-induced pulmonary vascular injury is a complication of T2DM that has been overlooked by researchers attempting to devise treatments for the numerous complications associated with the disease. Obesity, smoking, vascular disease, and prolonged diabetes are also significant contributors to reductions in lung function, and ex-smokers display clinically significant chronic air flow obstruction [6].
Colour doppler ultrasound (CDU), a modality that is widely used in a variety of medical fields, evaluates blood flow velocity based on shifts in Doppler signals [7]. Patients with hyperglycaemia and uncontrolled blood pressure have significantly increased blood flow compared to patients with systemic hypertension without diabetes (as estimated by CDU) [8]. The Doppler RI [PSV − PED/ PSV] that reflects intrarenal vascular resistance has been widely used to quantify the alterations in renal blood flow that may develop in renal disease [9].
To the best of our knowledge, no studies have used the intrarenal haemodynamics (RI) to predict changes in pulmonary function and intrarenal haemodynamics in patients with T2DM without DKD.
Methods
Subjects
The patients were included in the study: 1) patients with a diagnosis of T2DM, as defined by the guidelines of the American Diabetes Association [10]; 2) patients with no history of smoking, pulmonary disease, or recent viral illnesses; 3) patients without hepatopathy, nephropathy, hyperuricaemia, or gastrointestinal disease; and 4) patients likely to comply with the guidelines of the study who were able to visit our hospital for periodic assessments. The patients were excluded from the study: 1) patients with T1DM, as well as patients with gestational diabetes and patients who were lactating; 2) patients with inadequate renal function and other renal conditions that may affect intrarenal haemodynamics, such as urolithiasis, urinary infections, and large renal cysts; 3) patients whose liver function were abnormal or with the class III or IV heart failure or a history of coronary angioplasty, or myocardial infarction within 6 months before enrolling in the study; 5) patients with DKD and hypertension (patients receiving antihypertensive drugs).
Study design
154 adult patients with T2DM (82 males and 72 females) were initially diagnosed with the disease in 2000 and were subsequently divided into two separate groups according to their RIs (≤0.7 or >0.7); however, we were able to locate only 60 of these patients (32 males, 28 females) in 2015. These patients’ pulmonary functional parameters, RIs, UACRs, AERs, and BUN and Cr levels were measured in 2015 and compared to corresponding values measured in 2000.
Study assessments and endpoints
Blood specimen collection and laboratory testing: Venous blood samples were collected between 6 and 8 AM following a fasting period of at least 8 hours and used for measurements of FPG levels, HbA1c levels, and renal functional parameters (BUN, Cr). For this procedure, 5 ml of venous blood was placed in a glass tube, stored at room temperature for at least 10 min, and then centrifuged (3000 r/min) for 10 min to separate the plasma from the serum, the latter of which was stored at -70 °C in a cryogenic refrigerator. BUN and Cr levels were measured according to the instructions of the corresponding research kits. All the specimens were used for measurements of the above parameters within 1 week of collection.
Urine sample collection and laboratory tests: Urine output was quantified via a single 24-h urine collection. Urinary albumin concentrations were measured using a double-antibody radio immunoassay with a sensitivity of 0.5 mg/l, an intra-assay coefficient of variation of 4.5%, and an inter assay coefficient of variation of 5.3% within the range of 10-50 mg/l.
Pulmonary function measurements: The indicated pulmonary functional parameters (VC%, FVC%, FEV1%, PEF%, MVV%, TLC%, the FEV1/FVC% ratio, DLCO%, and the DLCO/VA% ratio) were measured using a spirometer. We used the measuredto- expected value ratios and the percentages of the predicted value to eliminate the influence of age, height, and weight on the results. The subjects were asked to remain seated and rest quietly for at least 30 min before testing. The pulmonary function tests were performed 3 times, and the best of 3 acceptable readings for each parameter was used in the analysis. Spirometry and pulmonary functional analysis were performed by trained professionals.
Intrarenal haemodynamic parameter measurements: We measured the indicated intrarenal haemodynamics parameters (PSV, EDV, and RI) in the bilateral interlobular renal arteries of subjects who had fasted for at least 8 h by Doppler sonography after documenting the subjects’ blood pressures and pulse rates. The examinations were performed after the subjects had rested for 15 min and with each subject in the supine position, as described previously. Colour duplex Doppler sonography was used to examine the vasculature of the left and right renal parenchyma and the main trunk of the renal artery. If no abnormalities in kidney size or vasculature structure were noted, 3 pulsed Doppler measurements were initiated in the interlobar arteries located at the center of the kidney, as well as in the arteries located at its upper and lower poles, within 5 minutes. The pulsed Doppler sampling gate was located in the interlobar arteries, and the angle of insonation, which was measured as precisely as possible, was kept under 60 °C. The PSV and EDV were documented in centimeters per second, and the RI was calculated as (PSV – EDV) / PSV. For each recording, the RI was measured only when at least 3 consecutive waveforms with similar appearances were observed. A mean of 3 RI measurements was obtained for each kidney. All examinations were performed in duplicate by the same operator, who had no knowledge of the two groups (diabetes or control) [11, 12].
Statistical analysis
Measurement data were expressed as the mean ± SD, and numerical data were expressed as percentages. Statistical analysis was conducted using the SPSS statistical package (Version 17.0, SPSS Inc. Chicago, IL, USA). Differences in categorical variables between the two groups were evaluated using the chi-square test; differences in continuous variables between the two groups were evaluated using the independent-samples t test; before and after treatment within-group differences in continuous variables were assessed using the paired-sample t test. P<0.05 was considered statistically significant.
Results
Pulmonary and renal function were assessed in patients with T2DM who were organized into separate groups according to their RIs. Subjects with RIs ≤0.7 had significantly better pulmonary (VC%, FVC%, FEV1%, PEF%, MVV%, TLC%, the FEV1/FVC% ratio, DLCO%, and the DLCO/VA% ratio), renal function (the AER and UACR and Cr, and BUN) in 2000 than subjects with RIs >0.7. Moreover, subjects in the former group displayed improvements and smaller decreases and increases in their pulmonary and renal functional parameters between 2000 and 2015, respectively, compared to subjects in the latter group. Additionally, the incidence of DKD in the group with RIs ≤0.7 (6/25, 24.00%) was significantly lower than that in the group with RIs >0.7 (23/35, 65.71%) (P<0.05) (Table 1).
Discussion
The results of this study indicate that in addition to renal functional parameters, the combination of the right kidney RI and the GFR may also be a good predictor of changes in pulmonary function in patients with diabetes, as well as a more sensitive indicator of changes in pulmonary function in such patients during the pre-clinical stages of DKD than the UACR or AER.
Renal Doppler RIs are widely used to evaluate blood flow in renal parenchymal diseases. RIs, which reflect intrarenal vascular resistance, a measure of vascular compliance [9], are also widely used to quantify changes in renal blood flow that may be attributable to renal diseases. Interestingly, previous studies have shown that renal Doppler sonography is an effective noninvasive and inexpensive means of screening for renovascular hypertension correctable via treatment with captopril [13]; therefore, we evaluated intrarenal haemodynamics by examining the RI using Doppler sonography.
The mechanisms underlying the occurrence of lung damage in diabetes are not fully understood. Thus, hyperglycaemia damages the lung, collagen is less susceptible to proteolysis because of nonenzymatic glycosylation of proteins in the lungs and chest wall, leading to its accumulation in lung connective tissue. This process is triggered mainly by hyperglycaemia and is thus more pronounced in patients with poor glycaemic control than in patients with good glycaemic control. In addition, nonenzymatic glycosylation of proteins in the lungs decreases lung compliance [14,15]. Clinically, loss of microvascular reserve in the lung may be associated with an increased risk of hypoxia in acute or chronic pathological lung conditions, such as pneumonia, asthma, chronic obstructive pulmonary disease, and congestive heart failure. Moreover, microvascular abnormalities frequently contribute to histological changes in the lung parenchyma, such as nodular fibrosis [15].
Systemic inflammation is another concern in patients with diabetes. Systemic inflammation induced by oxidative stress is associated with endothelial dysfunction in patients with diabetes [16-18]. Additionally, insulin resistance can alter lung volume and mechanical function via mediators such as leptin [15] and may independently cause airflow obstruction in a manner similar to that in which peripheral airway inflammation causes air flow obstruction in asthma [19]. Lung CO transfer capacity is significantly affected by the integrity of the lung capillary endothelium, a finding that supports that idea that clinicians should devote more attention to pulmonary vascular changes. The reports on lung function testing in patients with diabetes that have been published during the last 15 years have focused predominantly on pulmonary microangiopathy. The lung functional parameters that are related specifically to pulmonary microangiopathy include pulmonary capillary blood volume and CO transfer capacity [20]. Niranjan V found that TLC, FVC, FEV1, and VC values were significantly lower in patients with type 1 diabetes than in healthy subjects [21]. The results pertaining to the correlations between HbA1c and pulmonary function that were noted in previous studies were inconsistent. Two studies noted weak associations between HbA1c and spirometric parameters and strong correlations between diabetes duration and pulmonary function [22,23]. Another cross-sectional population study noted that plasma glucose levels were negatively correlated with FVC and/or FEV [24].
The precise mechanisms underlying DKD development are unknown; however, several theories exist regarding the specific processes that affect haemodynamics in DKD. Haemodynamic changes in diabetic kidneys have been the focus of considerable research. Renin-angiotensin system (RAS) activation reportedly
induces intrarenal haemodynamic abnormalities in diabetes [25]. Taniwaki H and colleagues demonstrated that the intrarenal RAS may be activated in diabetes and subsequently facilitate increases in the RI and that RAS activation may be impacted by poor glycaemic control. In addition, these authors showed that blocking RAS activation with captopril may reduce intrarenal vascular resistance in diabetes [26].
Elevated RIs have been reported to be associated with vascularinterstitial diseases, including DKD (but not glomerulopathies). Increased RIs may be reflective of decreased tissue and vascular compliance, as well as increased vascular resistance [9]. However, the early stages of DN are associated with an increased GFR and variable increases in renal plasma flow and the filtration fraction in both clinical and experimental settings. Diabetic hyper perfusion and hyperfiltration at the nephron level are characterized by disproportionate decreases in afferent arteriolar resistance. These changes may also be reflected by increased RIs. RIs are measured by duplex Doppler sonography [27]. Biopsy studies involving children have shown that basement membrane thickening and mesangial expansion in the kidney develop prior to the onset of microalbuminuria [28]. Doppler sonography apparently does not replace renal biopsy but is a readily applicable and noninvasive tool for investigating renal haemodynamics and a credible means of exploring renal structures for the purpose of collecting both morphologic and physiologic data for the study of renal blood flow in children [29]. To our knowledge, our study is the first to use the RI to predict changes in renal function in the preclinical stage of DKD, results similar to those of the study by Pelliccia P, which involved children [29].
However, there is no general agreement with respect to the significance and predictive value of the renal RI in patients with DKD. Researchers have performed several studies regarding the application of Doppler sonography for the evaluation of intrarenal haemodynamic abnormalities in adults with DKD [9,30]; however, studies regarding the preclinical stage of DKD (in which renal function is normal) in adults are still lacking. In our study, we aimed to explore whether Doppler sonography could be used to detect alterations in the renal RI in adults with diabetes who had normal renal function.
Table 1: The change of RI in the interlobular renal arteries and the pulmonary function from baseline to year 15.
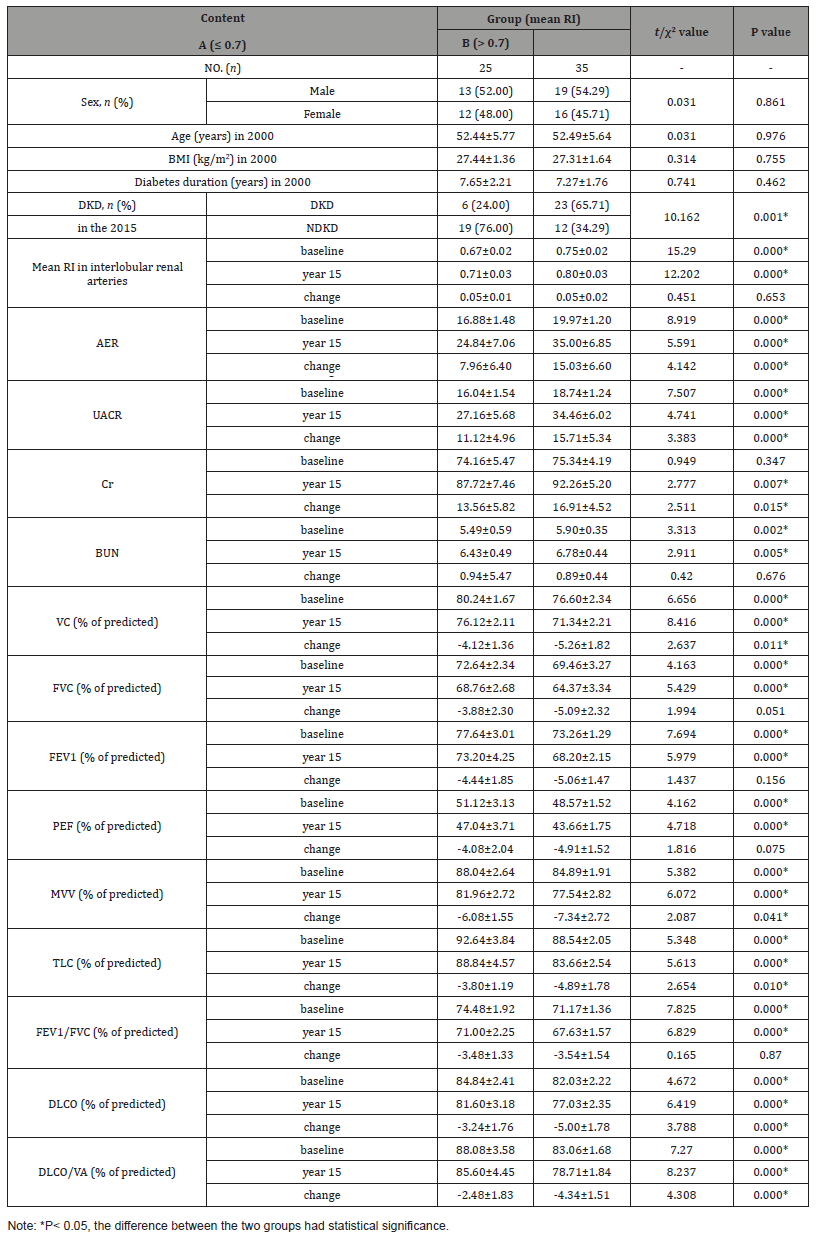
In our study, the results of the longitudinal follow-up study, in which pulmonary function was assessed in patients organized into separate groups according to their RIs, indicated that subjects with RIs ≤0.7 displayed significantly better pulmonary function in 2000 than subjects with RIs >0.7. Moreover, pulmonary function showed improvement and decreased to a smaller extent in the former group compared to the latter group from 2000 to 2015. Additionally, the indicated renal functional parameters (BUN and Cr) and the AER and UACR showed improvement and increased to a smaller extent in the former group compared to the latter group from 2000 to 2015. The incidence of DKD in the group with RIs ≤0.7 (6/25, 24.00%) was significantly lower than that in the group with RIs >0.7 (23/45, 65.71%) (P<0.05) (Table 1).
In our study, we demonstrated that pulmonary functional parameters were negatively correlated with the bilateral kidney RI; however, our study had several limitations that should be addressed in future studies. First, we failed to observe the changes in alveolar tissue morphology associated with diabetes and did not identify the specific protein responsible for inducing the changes. Because not all the patients underwent a lung biopsy, we had to adopt an animal model to study alveolar tissue samples. Second, we did not assess inflammatory factors or ROS, both of which are associated with lung dysfunction in T2DM [16-18]. It is worth evaluating whether they can be used to predict pulmonary function and renal function during the preclinical stage of DKD. Therefore, we recommend that clinicians monitor patients with T2DM for signs of lung damage in addition to monitoring them for signs of DKD and DR.
Conclusion
In conclusion, According to the above results, we surmised that the kidney RI may be used not only to evaluate the haemodynamic changes characteristic of DKD and the progression of the disease but also to predict pulmonary and renal function in adults and children during the preclinical stage of DKD. Thus, in addition to the HbA1c and diabetes duration, the renal RI, can also be used to predict the likelihood of DKD during the preclinical stage of the disease in question.
Acknowledgement
The authors would like to thank all of the patients and their families, the team of investigators, research nurses, and operations staff involved in this study. Editorial support (in the form of writing assistance, including development of the initial draft based on author input, assembling tables and figures, collating authors comments, grammatical editing and referencing) was provided by He Tai. The translator of English was provided by American journal experts.
Availability of Data and Materials
The data from the original study are available from Shenyang the Fourth Hospital of People via its ClinicalTrials.gov (NCT02798198). The datasets analyzed during the current study are available from all the authors on reasonable request.
Ethics Approval and Consent to Participate
The study complied with the Declaration of Helsinki and the International Conference on Harmonisation and Good Clinical Practice guidelines. The protocol was approved by the regulatory authority for each country (where applicable) and an independent ethics committee at each center. and the clinical research protocol was approved by the medical ethics committee (number ICE20160806) of our hospital. Each subject who participated in the study or one of their family members provided written informed consent authorizing their enrolment herein.
Conflict of interest
Author declare no conflict of interest.
References
- Pedroza Tobias AL, Hernandez Barrera N, Lopez Olmedo A, Garcia Guerra S, Rodriguez Ramirez I, et al. (2016) Usual Vitamin Intakes by Mexican Populations. J Nutr 146(9): 1866-1873.
- Bojar I, Owoc A, Humeniuk E, Fronczak A, Walecka I (2014) Quality of pregnant women's diet in Poland-macro-elements. Arch Med Sci 10(2): 361-365.
- Ervin PA, Bubak V (2019) Closing the rural-urban gap in child malnutrition: Evidence from Paraguay, 1997-2012. Econ Hum Biol 32: 1-10.
- Liu H, Fang H, Zhao Z (2013) Urban-rural disparities of child health and nutritional status in China from 1989-2006. Econ Hum Biol 11(3): 294-309.
- Liu H, Rizzo JA, Fang H (2015) Urban-rural disparities in child nutrition-related health outcomes in China: The role of hukou policy BMC Public Health 15: 1159.
- Sharaf MF, Mansour EI, Rashad AS (2019) Child nutritional status in egypt: a comprehensive analysis of socioeconomic determinants using a quantile regression approach. J Biosoc Sci 51(1): 1-17.
- Zhang N, Becares L, Chandola T (2016) Patterns and Determinants of Double-Burden of Malnutrition among Rural Children: Evidence from China. PLoS One 11(7): e0158119.
- Mabogunje A (1968) Urbanization in Nigeria. University of London Press, London.
- Adedini SA, Odimegwu C, Imasiku ENS, Ononokpono DN (2015) Ethnic differentials in under-five mortality in Nigeria. Ethn Health 20(2): 145-162.
- Cuttler R, Evans R, McClusky E, Purser L, Klassen KM, et al. (2019) An investigation of the cost of food in the Geelong region of rural Victoria: Essential data to support planning to improve access to nutritious food. Health Promotion Journal of Australia 30(1): 124-127.
- Palermo C, McCartan J, Kleve S, Sinha K, Shiell A (2016) A longitudinal study of the cost of food in Victoria influenced by geography and nutritional quality. Aust N Z J Public Health 40(3): 270-273.
- Rossimel A, Han SS, Larsen K, Palermo C (2016) Access and affordability of nutritious food in metropolitan Melbourne. Nutrition & Dietetics 73(1): 13-18.
- Williams P (2010) Monitoring the Affordability of Healthy Eating: A Case Study of 10 Years of the Illawarra Healthy Food Basket. Nutrients 2(11): 1132-1140.
- Ashton MM, Dean OM, Marx WG, Mohebbi M, Berk M, et al. (2020) Diet quality, dietary inflammatory index and body mass index as predictors of response to adjunctive N-acetylcysteine and mitochondrial agents in adults with bipolar disorder: A sub-study of a randomised placebo-controlled trial. Aust N Z J Psychiatry 54(2): 159-172.
- Cantoni M, Paffenbarger RS, Krueger DE (1961) Methods of dietary assessment in current epidemiologic studies of cardiovascular diseases. Am J Public Health Nations Health 51: 70-75.
- Blaine RE, Kachurak A, Davison KK, Klabunde R, Fisher JO (2017) Food parenting and child snacking: a systematic review. Int J Behav Nutr Phys Act 14(1): 146.
- Burnett AJ, Worsley A, Lacy KE, Lamb KE (2019) Moderation of associations between maternal parenting styles and Australian pre-school children's dietary intake by family structure and mother's employment status. Public Health Nutr 22(6): 997-1009.
- Haines J, Haycraft E, Lytle L, Nicklaus S, Kok FJ, et al. (2019) Nurturing Children's Healthy Eating: Position statement. Appetite 137: 124-133.
- Llanaj E, Adany R, Lachat C, Haese MD (2018) Examining food intake and eating out of home patterns among university students. PLoS One 13(10): e0197874.
- Scaglioni S, Cosmi De V, Ciappolino V, Parazzini F, Brambilla P, et al. (2018) Factors Influencing Children's Eating Behaviours. Nutrients 10(6): E706.
- Choy CC, Desai MM, Park JJ, Frame EA, Thompson AA, et al. (2017) Child, maternal and household-level correlates of nutritional status: a cross-sectional study among young Samoan children. Public Health Nutr 20(7): 1235-1247.
- Kosaka S, Suda K, Gunawan B, Raksanagara A, Watanabe C, et al. (2018) Urban-rural difference in the determinants of dietary and energy intake patterns: A case study in West Java, Indonesia. PLoS One 13(5): e0197626.
- Liu HQ, Hall JJ, Xu XY, Mishra GD, Byles JE (2018) Differences in food and nutrient intakes between Australian- and Asian-born women living in Australia: Results from the Australian Longitudinal Study on Women's Health. Nutr Diet 75(2): 142-150.
- Mattei J, Malik V, Wedick NM, Hu FB, Spiegelman D, et al. (2015) Reducing the global burden of type 2 diabetes by improving the quality of staple foods: The Global Nutrition and Epidemiologic Transition Initiative. Global Health 11: 23.
- Sievert K, Lawrence M, Naika A, Baker P (2019) Processed Foods and Nutrition Transition in the Pacific: Regional Trends, Patterns and Food System Drivers. Nutrients 11(6): E1328.
- Zeba AN, Yameogo MT, Tougouma SJB, Kassie D, Fournet F (2017) Can Urbanization, Social and Spatial Disparities Help to Understand the Rise of Cardiometabolic Risk Factors in Bobo-Dioulasso? A Study in a Secondary City of Burkina Faso, West Africa. Int J Environ Res Public Health 14(4): E378.
- Galgani J, E Ravussin (2008) Energy metabolism, fuel selection and body weight regulation. Int J Obes (Lond) 32: 109-119.
- Doucet E, Tremblay A (1997) Food intake, energy balance and body weight control. Eur J Clin Nutr 51(12): 846-855.
- Mendoza JA, Drewnowski A, Christakis DA (2007) Dietary energy density is associated with obesity and the metabolic syndrome in US adults. Diabetes Care 30(4): 974-979.
- Ebbeling CB, Feldman HA, Klein GL, Wong JMW, Bielak V, et al. (2018) Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ 14: 363.
- Martens EA, Gonnissen HK, Gatta Cherifi B, Janssens PL, Westerterp Plantenga MS (2015) Maintenance of energy expenditure on high-protein vs. high-carbohydrate diets at a constant body weight may prevent a positive energy balance. Clin Nutr 34(5): 968-975.
- Veldhorst MA, Westerterp KR, Van Vught A, Westerterp Plantenga MS (2010) Presence or absence of carbohydrates and the proportion of fat in a high-protein diet affect appetite suppression but not energy expenditure in normal-weight human subjects fed in energy balance. Br J Nutr 104(9): 1395-1405.
- Westerterp Plantenga MS, Lemmens SG, Westerterp KR (2012) Dietary protein - its role in satiety, energetics, weight loss and health. Br J Nutr 108: S105-112.
- Hall KD, Ayuketah A, Brychta R, Cai HY, Cassimatis T, et al. (2019) Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab 30(1): 67-77.
- Bartelt A, Weigelt C, Cherradi ML, Niemeier A, Thodter K, et al. (2013) Effects of adipocyte lipoprotein lipase on de novo lipogenesis and white adipose tissue browning. Biochim Biophys Acta 1831(5): 934-942.
- Grobe JL, Venegas Pont M, Sigmund CD, Ryan MJ (2009) PPAR gamma differentially regulates energy substrate handling in brown vs. white adipose: focus on "The PPAR gamma agonist rosiglitazone enhances rat brown adipose tissue lipogenesis from glucose without altering glucose uptake. Am J Physiol Regul Integr Comp Physiol. 296(5): R1325-R1326.
- Potter JL, Collins CE, Brown LJ, Hure AJ (2014) Diet quality of Australian breast cancer survivors: a cross-sectional analysis from the Australian Longitudinal Study on Women's Health. J Hum Nutr Diet 27(6): 569-576.
- Tsigga M, Filis V, Hatzopoulou K, Kotzamanidis C, Grammatikopoulou MG (2011) Healthy Eating Index during pregnancy according to pre-gravid and gravid weight status. Public Health Nutr 14(2): 290-296.
- Gardner CD (2019) Preventing weight gain more important than weight loss and more realistic to study in cohorts than in randomized controlled trials. Am J Clin Nutr 110(3): 544-545.
- Lombard CB, Harrison CL, Kozica SL, Zoungas S, Keating C, et al. (2014) Effectiveness and implementation of an obesity prevention intervention: The HeLP-her Rural cluster randomised controlled trial.” BMC Public Health 14: 608.
- (NPC) NPC (2014) [Nigeria] 2006 Population and Housing Census of the Federal Republic of Nigeria. Abuja, Nigeria: National Population Commission; 2006.” NPC (2): 236-651.
- Kaviani S, Van Dellen M, Cooper JA (2019) Daily Self-Weighing to Prevent Holiday-Associated Weight Gain in Adults. Obesity (Silver Spring) 27(6): 908-916.
-
Lian Qun Jia, Xiao Lin Jiang, Wei Chen, He Tai, Jin Song Kuang. Longitudinal follow-up Study of the Intrarenal emodynamics and Pulmonary Function in Patients with T2DM. On J Complement & Alt Med. 3(4): 2020. OJCAM.MS.ID.000570.
-
Dietary food, Secondary school, Protein, Household activities, Food frequency questionnaire, Root tuber carbs, Weight gain, Socioeconomic environment, Diet-related diseases
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






