 Review Article
Review Article
Dynamics of The Universal Test and Treat Strategy in HIV Patients in Nigeria: A Narrative Review of Studies
Nwankwo Gloria Ogochukwu1, Ogbonna Brian Onyebuchi1,2*, Anetoh Maureen Ugonwa1, Ejieh Loveth1, Adenola Ugochi Amanda1, Okpalanma Nneoma3, Maduekwe Hilda3, Okeke Anthony4, Okoye Ifunanya3, Omuta Michael Chukwuemeka4, Egere Eustace Chijioke4, Osuafor Nkeiruka Grace4, Maduka Anthony4, Ovwighose Samuel4, Nnamani Monica4, Ajagu Nnenna5 and Ofor Amala5
1Department of Clinical Pharmacy and Pharmacy Management, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka, Nigeria
2Department of Clinical Pharmacy and Pharmacy Practice, Faculty of Pharmacy, King David University of Medical Sciences, Uburu, Nigeriaa
3Department of Clinical Pharmacy and Pharmacy Management, Faculty of Pharmaceutical Sciences, Chukwuemeka Odimegwu Ojukwu University Igboariam Nigeria
4Department of Clinical Pharmacy and Pharmacy Management, Faculty of Pharmaceutical Sciences, Madona University, Elele Nigeria
5Department of Clinical Pharmacy and Pharmacy Management, Faculty of Pharmaceutical Sciences, Enugu State University of Sience and Technology, ESUTEnugu
Ogbonna Brian O, Department of Clinical Pharmacy and Pharmacy Management, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka, Nigeria. Department of Clinical Pharmacy and Pharmacy Practice, Faculty of Pharmacy, King David University of Medical Sciences, Uburu, Nigeria.
Received Date:June 15, 2022; Published Date:July 22, 2022
Abstract
HIV and AIDS infection are major public health problems in sub-Saharan Africa. HIV is spread through contact with certain body fluids from a person with HIV, such body fluids include; blood, semen, pre-seminal fluid, vaginal fluids, rectal fluids, and breast milk. HIV attacks and destroys the CD4 cells. Antiretroviral Therapy (ART) is recommended for everyone who has HIV. ART prevents the HIV from multiplying and reduces the amount of HIV and viral load to an undetectable level in the body. According to the latest HIV treatment Guideline Nigeria published in 2016, it says; ART should be initiated in all adults, adolescents, pregnant and breastfeeding women, and children with a diagnosis of HIV regardless of WHO clinical stage and regardless of CD4+ cell count. Initiating ART treatment as soon as possible after HIV infection has been demonstrated to reduce viral load, decrease transmission and possibly reduce rates of loss to follow-up (LTFU) patients. This study assessed the attributes and outcomes of the universal test and treat strategy in HIV patients in Nigeria. This study reviewed the overview of HIV patients in Nigeria after the implementation of the Universal test and treat strategy initiated in 2016 with the use of a narrative style of literature review. This study reviewed literature retrieved from searches of computerized databases. The studies selected included those written in the English Language that was carried out in Nigeria with a clear study design and properly stated year of publication which fell within the stated years of 2016 to 2021. A total of 17 articles met the study criteria and were used for the study. South-west (29.41%) had the highest distribution of articles on HIV test and treat strategy done in Nigeria. This was followed by South-south with a distribution of (17.65%). North-west, North-central, South-west and Nationwide had an equal distribution of (11.76%). North-east had the smallest number of studies done so far in Nigeria with (5.88%). Most of the studies were conducted between 2011- 2020 and three articles were published in 2021 (17.65%). The articles cited in this study are those of HIV patients of major population groups who are receiving ART care with the new treatment guidelines in Nigeria. The distribution of the studies conducted on the HIV test and treat strategy was more in the South-west region of Nigeria than in other geopolitical regions.
Keywords:Universal test and treat strategy; HIV infection; Nigeria; Antiretroviral
Abbreviations:HIV: Human Immunodeficiency Virus; AIDS: Acquired Immune Deficiency Syndrome; ART: Anti-retroviral Therapy; UTT: Universal test and treat; LTFU: Lost to follow up; PLHIV: People Living with HIV
Introduction
HIV (Human Immunodeficiency Virus) remains a world public health problem and Nigeria is not left out. Nigeria has the secondlargest HIV epidemic globally [1]. Antiretroviral Therapy (ART) prevents the HIV from multiplying and reduces the amount of HIV and viral load to an undetectable level in the body. The advent of Antiretroviral Therapy (ART) has brought about a reduction in the mortality rate of HIV, although it is still a thing of great concern because the number of people living with HIV/AIDS (PLWHA) still increases every year. Late initiation of antiretroviral therapy, high patient attrition between HIV testing and, treatment initiation and loss of follow-up is a persistent challenge in HIV care and have posed significant threats to the success of the ART program [2].
World Health Organization (WHO) developed the “universal test and treat” (UTT) program as a strategy for HIV elimination in place of the previous “differed treatment” (CD4 based and WHO clinical staging approaches) program [3]. The Federal Ministry of Health in Nigeria thereby updated its HIV treatment guidelines in December 2016 to the Universal Test and Treat approach. This program encouraged and recommended all populations at risk to be screened for HIV infection and for those diagnosed HIV positive to receive early treatment regardless of their CD4+ cell count and WHO clinical stage and recommended that ART be initiated within two weeks of HIV diagnosis. Initiating ART treatment as soon as possible after HIV infection has been demonstrated to reduce viral load and decrease transmission [4].
Expanded guidelines are critical to getting more patients onto HIV treatment, but the increasing numbers of patients eligible for treatment could negatively impact clinics if they cannot keep up with demand [5]. Healthcare providers may experience a strain that might reduce the quality of services rendered to patients due to an increase in patient volume. It could also negatively impact the purpose of the program for the patients because those patients with high CD4+ cell count might not see the early ART initiation as a priority leading to attrition from care and then a loss of follow-up [6].
.Loss of follow-up among patients enrolled in antiretroviral therapy for HIV/AIDS is a persistent challenge in sub-Saharan Africa [7]. Continuous care is a key factor for the effectiveness of antiretroviral treatment and for achieving maximum targets. Patients lost to follow-up (LTFU) in the treatment plan poses a great risk and huge problem to the general public and health care services because such patient is at a greater risk of transferring the virus to other patients than a patient who is taking their antiretroviral treatments. There is also a risk that LTFU patients will discontinue or interrupt ART. These treatment interruptions are concerning as they may lead to viral rebound and an increased chance of HIV transmission and drug resistance [8]. This study reviewed the overview of HIV patients in Nigeria after the implementation of the Universal test and treat strategy initiated in 2016 with the use of a narrative style of literature review.
Materials and Methods
Study Area:The study covered the universal test and treat strategies of HIV patients’ studies carried out in Nigeria.
Review question:What are the dynamics and outcomes of the universal test and treat strategy for HIV patients in Nigeria?
Study population and type of studies included:The search was carried out on PubMed and Google Scholar for studies that met the inclusion criteria. This ensured retrieval of relevant studies while focusing on the study objectives.
Eligibility criteria
Inclusion criteria
Studies on the outcomes of HIV patients after the test and treat
strategy conducted in Nigeria irrespective of the region
• Studies published in the English language
• Peer-reviewed papers were eligible for inclusion
• Studies with defined protocol and study design either
experimental or non-experimental
• Studies with no conflict of interest stated
• Studies that provided other information that may help to
understand HIV patients’ outcome
• Studies with clearly stated and defined research design.
Exclusion Criteria
• Related studies without a clearly defined period, duration,
sample size, and location were discarded
• Related studies with methodological flaws
• Studies with incomplete data.
Study design:The study was a narrative overview of the dynamics of the universal test and treat strategy in HIV patients in Nigeria.
Risk of Bias:The included studies were assessed for subjects and sampling selection bias, reporting bias before selection.
Condition and Domain studied:HIV care studies after the UTT implementation and articles that described HIV patients’ outcomes in Nigeria.
Information source:Search was conducted using Google Scholar and PubMed. Data extraction was done by the standard reporting protocol for narrative reviews [9].
Data items:Articles that met the inclusion criteria were selected and the relevant data were abstracted and checked to ensure quality consistency. Relevant variables obtained from articles include; the title of publication, study location, sample size, study design, year of publication, inclusion criteria, exclusion criteria, and study instrument. In any case of missing data, the article was excluded.
Context:The study covered the outcomes of HIV patients on the universal test and treat strategy studies carried out in Nigeria from 2015 to 2021.
Articles search process:The keywords related to the title of the study was used for the search. PubMed and Google Scholar were used to search for studies and articles on HIV care after the treat-all strategy in Nigeria and were published between 2015 and 2021. Free-text search terms and Medical Subject Headings (MeSH) used for the article search included; “test and treat”, “HIV treatment”, “outcome” “Antiretroviral”, “retention”, “attrition”, and “Nigeria”. Additional words found appropriate and relevant to the title and objective of the study were utilized. A total of 747 articles were obtained, 530 articles from Google Scholar, and 217 came from PubMed. These articles were assessed for eligibility based on the inclusion criteria. The figure below (Figure 1) represents a graphical illustration of how the search was conducted.

Study period and duration:The study lasted from October to December 2021 and covered peer-reviewed articles published from January 2015 to December 2021.
Ethical approval:Ethical approval is not applicable here. However, only studies with ethical approval were included and utilized in the review process.
Data analysis:Descriptive analysis of percentage, proportion, and frequency distribution was carried out for all the studies included.
Study articles selection process:Overall, 747 articles were obtained, (217 from PubMed and 530 from Google Scholar). The articles obtained were thoroughly assessed for eligibility based on the inclusion criteria. On screening, 238 articles were removed because they were duplicates. Further screening identified 456 articles outside the scope of outcomes of HIV patients and was discarded giving 53 articles. And another 36 articles were discarded due to invalid study design and incomplete follow-up data finally giving rise to 17 articles that were used for the review.
Data extraction instrument, pilot testing, and data extraction process
Data included in this study were carefully extracted by a thorough consideration of the articles, elimination of those that did not meet the study objective and criteria, and elimination of irrelevant or incomplete articles. The data extraction design was adapted from a similar study carried out in Nigeria by Ogbonna et al. [10]. The remaining data that passed the inclusion criteria were analyzed and pilot tested. Five articles were used for the pilot test and they were not included in the study. The final instrument was obtained by proper arrangement and designing of the data items into an appropriate table format. The instrument was approved by an independent assessor after critiquing it by applying it to two independent studies before being used for the data collection.
Table 1:Evidence -based table of the study articles.
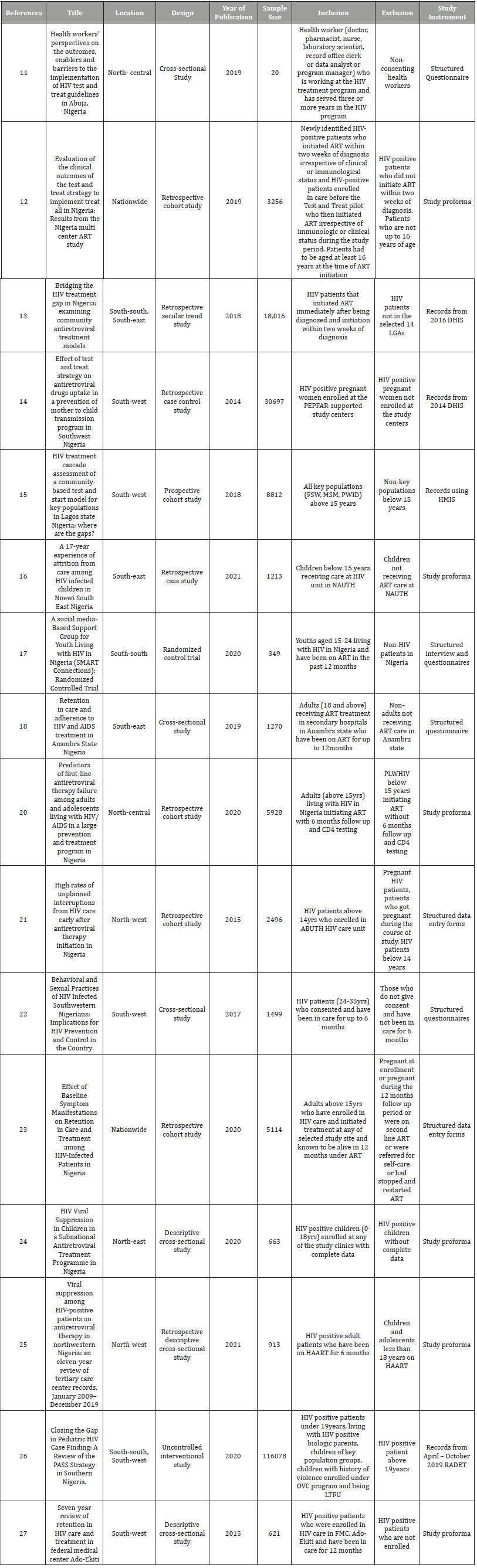
Table 2:Focus on studies on HIV test and treat Strategy in Nigeria according to geo-political zone distribution.
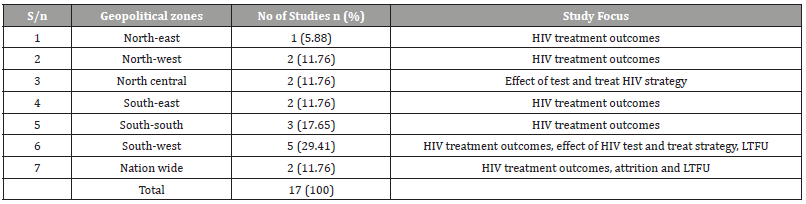
Table 3:Assessment of Studies on HIV test and treat Strategy in Nigeria based on Oxford Center for Evidence-Based Medicine’s Levels of Evidence from Highest to Lowest [9].
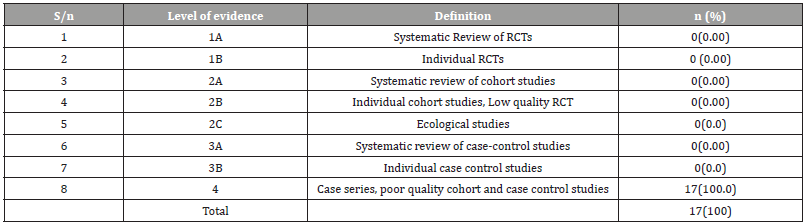
Table 4:Assessment of HIV test and treat Strategy studies on in Nigeria based on the Scottish Intercollegiate Guidelines Network for hierarchy of Study Type [28].
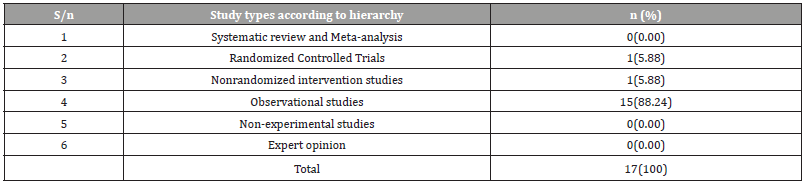
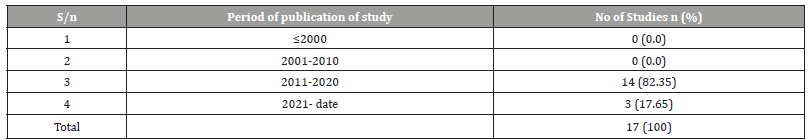
Table 5:Periodic Distribution of HIV test and treat Strategy studies in Nigeria.
Discussion
An Overview of Universal Test and Treat Strategy in HIV patients in Nigeria
An estimated 3.2 million people are living with HIV/AIDS in Nigeria and only about one-third of this number have access to ART [12]. The availability of antiretroviral treatment to HIV patients has helped with patient survival, reduction of HIV transmission, and generally a better quality of life [18]. The universal test and treat strategy are aimed at getting some patients on ART once they test positive regardless of the CD4+ count [12]. Studies have also demonstrated that initiating ART immediately after a positive HIV test could help with control of the disease epidemic and invariably lead to better outcomes [29]. This prompted the World Health Organization to launch the Treat All guidelines in 2016 [30]. The United States President’s Emergency Plan for AIDS Relief (PEPFAR) in Nigeria, supported the HIV treatment centers in Nigeria to pilot the Universal Test and Treat Strategy by initiating Anti-Retroviral Therapy in HIV-positive patients within two weeks of diagnosis in October 2016 [31].
In December 2016, the Federal Ministry of Health of Nigeria made two vital changes to the Standard Treatment Guidelines for HIV to expand the Treat All strategy to all HIV treatment sites nationwide and to reduce the time gap between diagnosis of HIV and treatment. The two significant changes include; ART should be initiated immediately after a positive HIV diagnosis or within two weeks and ii) the CD4 count threshold was removed to allow ART eligible for all HIV-positive patients [31]. This new policy is against the previous 2010 edition of the HIV STG Nigeria that states that initiation of therapy at CD4 counts of ≤350 cells/mm3 for all HIVpositive individuals irrespective of gender [33], and this strategy has been said to control the epidemic to some extent [2].
Since the beginning of large-scale antiretroviral availability early in this decade, ART programs in Africa have retained about 60% of the patients at the end of the second year meaning that there has been a decline in HIV-related morbidity, mortality, and new HIV infections globally [37]. Patients don’t have to make multiple visits to the clinics and do multiple CD4 cell counts with long repeated counseling without initiating treatments. The previous treatment guideline could be perceived by the patient yet started on ART as unnecessary and time-wasting [32].
Late initiation of antiretroviral therapy, high patient attrition between HIV testing and treatment initiation and loss to followup is persistent challenges in HIV care and have posed significant threats to the success of the ART program [2]. Generally, patients are classified as loss of follow up if, at least 60 days had elapsed since the patient’s last scheduled pick-up date and they did not later return. Patients who died, withdrew or transferred out of the facility are not considered a loss to follow up [35]. For a patient to achieve viral suppression, he or she must be retained in care which includes services and cares from HIV diagnosis to a lifetime [18].
With the recent World Health Organization’s recommendation of the test and treat strategy, there would be no need for pre-ART care whereby HIV patient’s ineligible for ART were monitored for disease progression. But instead, since all patients are initiated on ART almost immediately, it is expected that there would be better patient outcomes, but it is still limited due to high patient attrition and the high number of patients lost to follow-up. In a recent study conducted by Stafford et al. [1], it was found that approximately one in three HIV-positive patients in Nigeria who participated in the test and treatment pilot were LTFU by 1 year after treatment initiation when compared to one in five HIV patients who had been previously in HIV care [1].
However, with the new treatment guidelines, the newly diagnosed HIV patients who don’t undergo the pre-ART care included in the previous guidelines may not value the need for initiating ART because they have high baseline CD4 count and are probably asymptomatic. In addition to this, there has been a strain on health workers in the HIV clinic because more and more patients are now started up on ART every day without an increase in the number of health workers to attend to them leading to higher waiting-times in the clinic [11]. Most patients get discouraged by the time wasting and hence get lost in follow up [11]. These could be perceived as a major disadvantage with the test and treatment guidelines.
Ideally, once a patient test positive for HIV, he/she is initiated on ART, which means they must return for clinic visits, prescription drug refills, counseling, and laboratory tests. Most times, these requirements are a bit tasking and sometimes inconvenient, so the patients become prone to discontinue care leading to an Interruption in Treatment and creating a major challenge to the HIV program and the society as a whole [35].
Description of the HIV test and treat strategy and the extent of work done in Nigeria
From the data in Table 2, southwest had the highest number of studies conducted on the new HIV treatment guideline covering one-third of the total distribution. This was followed by Southsouth. North-west, North-central, South-west and Nationwide had an equal distribution of approximately twelve percent each. Northeast had the smallest number of studies done so far in Nigeria with (5.88%). Most of the studies gave an overview of the outcomes of the new HIV treatment guidelines in different regions, states, and populations. Studies conducted in the South-west focused more on patient attrition and loss of follow-up since these are the major challenges with PLHIV.
Conclusion
The articles cited in this study are those of HIV patients of major population groups who are receiving ART care with the new treatment guidelines in Nigeria. The distribution of the studies conducted on the HIV test and treat strategy was more in the Southwest region of Nigeria than in other geopolitical regions.
Limitations
The possibility of omission was due to search terms limitations. Some of the studies sited may have some level of bias which escaped elimination which could have an impact on the outcome of the study. The method of presenting tables and data in the present study was purposively chosen for simplicity and clarity even though they could be better presentation formats.
Acknowledgment
None.
Conflict of Interest
The authors declare no competing interests.
- Stafford KA, Odafe SF, Lo J, Ibrahim R, Ehoche A, et al. (2019) Evaluation of the clinical outcomes of the Test and Treat strategy to implement Treat All in Nigeria: Results from the Nigeria Multi-Center ART Study. PLoS ONE 14(7): e0218555.
- Fox MP, Rosen S, Geldsetzer P, Barnighausen T, Negussie E, Beanland R. (2016) Interventions to improve the rate or timing of initiation of antiretroviral therapy for HIV in sub-Saharan Africa: meta-analyses of effectiveness. J Int AIDS Soc 19(1): 20888.
- World Health Organization (2016) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2nd WHO, Geneva.
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365(6): 493-505.
- Mberi MN, Kuonza LR, Dube NM, Nattey C, Manda S, et al. (2015) Determinants of loss to follow-up in patients on antiretroviral treatment, South Africa, 2004–2012: a cohort study. BMC Health Serv Res 15: 259.
- Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, et al. (2014) Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis 14(4): 281-290.
- Agu KA, Isah MA, Oqua D, King RC, Wutoh AK (2012) Retention in HIV care among patients testing positive for HIV and ineligible to start antiretroviral therapy. World J AIDS 2(4): 330-337.
- Granich R, Crowley S, Vitoria M, Smyth C, Kahn JG, et al. (2010) Highly active antiretroviral treatment as prevention of HIV transmission: review of scientific evidence and update. Curr Opin HIV AIDS 5(4): 298-304.
- Nikolaos AP, Apostolos AA, John PA (2005) Relative citation impact of various study designs in the health sciences. JAMA 293(19): 2362-2366.
- Ogbonna BO, Opera AC, Odilia VU. (2019) Pharmaceutical Care Activities in Nigeria from 1970 to 2018: A Narrative Review. Ecronicon Pharmacology and Toxicology 7(8): 789-805.
- Odafe S, Stafford KA, Gambo A, Onotu D, Swaminathan M, et al. (2019) Health Workers’ Perspectives on the Outcomes, Enablers, and Barriers to the Implementation of HIV “Test and Treat” Guidelines in Abuja, Nigeria. J AIDS HIV Treat 1(2): 33-45.
- Stafford KA, Odafe SF, Lo J, Ibrahim R, Ehoche A, et al. (2019) Evaluation of the clinical outcomes of the Test and Treat strategy to implement Treat All in Nigeria: Results from the Nigeria Multi-Center ART Study. PLoS ONE. 14(7): e0218555
- Edward A Oladele, Okikiolu A Badejo, Christopher Obanubi, Emeka F Okechukwu, Ezekiel James, et al. (2018) Bridging the HIV treatment gap in Nigeria: examining community antiretroviral treatment models. J Int AIDS Soc 21(4): e25108.
- Abayomi JA, Isah H, Edet-Utan K, Akinmurele T (2014) Effect of Test- and -Treat Strategy on Antiretroviral Drugs Uptake in a Prevention of Mother to Child Transmission Programme in Southwest Nigeria. Science Journal of Public Health 2(5): 476-479.
- Njab J, Adebajo S, Eluwa G, Shoyemi E, Osakwe P, et al. (2018) HIV Treatment Cascade Assessment of a Community-Based Test and Start Model for Key Populations in Lagos State Nigeria: Where Are the Gaps? World Journal of AIDS 8: 105-117.
- Onubogu CU, Ugochukwu EF (2021) A 17-year experience of attrition from care among HIV infected children in Nnewi South East Nigeria. BMC Infectious Diseases 21: 409.
- Dulli L, Ridgeway K, Packer C, Murray KR, Mumuni T, et al. (2020) A social media-Based Support Group for Youth Living with HIV in Nigeria (SMART Connections): Randomized Controlled Trial. J Med Internet Res 22(6): e18343.
- Umeokonkwo CD, Onoka CA, Agu PA, Ossai EN, Balogun MS, et al. (2019) Retention in care and adherence to HIV and AIDS treatment in Anambra State Nigeria. BMC Infect Dis 19(1): 654.
- Kuhns LM, Johnson AK, Adetunji A, Kuti KM, Garofalo R, et al. (2021) Adaptation of evidence-based approaches to promote HIV testing and treatment engagement among high-risk Nigerian youth. PLoS One 16(10): e0258190.
- Ndembi N, Murtala-Ibrahim F, Tola M, et al. (2020) Predictors of first-line antiretroviral therapy failure among adults and adolescents living with HIV/AIDS in a large prevention and treatment program in Nigeria. AIDS Res Ther 17: 64.
- Honkhai AA, Banigbe B, Adeola J, et al. (2015) High rates of unplanned interruptions from HIV care early after antiretroviral therapy initiation in Nigeria. BMC Infect Dis 15: 397.
- Ezechi OC, David AN, Idigbe IE, Ohihoin AG (2017) Behavioral and Sexual Practices of HIV Infected Southwestern Nigerians: Implications for HIV Prevention and Control in the Country. Journal of Prevention & Treatment of HIV/AIDS 2(1): 1-5.
- Juliet A, Okikiolu AB, Aimalohi A, Prosper O, Patrick AA, et al. (2020) Effect of Baseline Symptom Manifestations on Retention in Care and Treatment among HIV-Infected Patients in Nigeria. J Int Assoc Provid AIDS Care 19: 2325958220903575.
- Isaac E, Ajani A, Iliya J, Christianah O, Hassan D (2020) HIV Viral Suppression in Children in a Subnational Antiretroviral Treatment Programme in Nigeria. World Journal of AIDS 10: 170-185.
- Abdullahi SB, Ibrahim OR, Okeji AB, Rabilu IY, Bashir I, et al. (2021) Viral suppression among HIV-positive patients on antiretroviral therapy in northwestern Nigeria: an eleven-year review of tertiary care center records, January 2009–December 2019. BMC Infect Dis 21(1): 1031.
- Moses K, Doreen M, Tessie P, Emnet A, Maryam B, et al. (2020) Closing the Gap in Pediatric HIV Case Finding: A Review of the PASS Strategy in Southern Nigeria. Int J Vrol AIDS 7: 068.
- Oluwoleadeyemi B, Olujide JO, Oladele AA, David SE, Adebusuyi OO, et al. (2015) Seven-year review of retention in HIV care and treatment in Federal Medical Center Ido-Ekiti. Pan Afr Med J 22: 139.
- Mann JJ, Apter A, Dianne C, Jose M, Ann H, et al. (2005) Suicide prevention strategies: A systematic review. JAMA 294(16): 2064-2074.
- Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. (2015) Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 373(9): 795-807.
- World Health Organization (2016) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. WHO; Geneva.
- (2016) National Guidelines for HIV Prevention, Treatment and Care. National AIDS and STIs Control Programme. Federal Ministry of Health Nigeria.
- Bayaki S, Dadja E, Akouda P, Stephane d’A, Assetina S, et. al. (2013) Loss of HIV-infected patients on potent antiretroviral therapy. Togo: risk factors and the fate of these patients. Pan Afr Med J 15: 35.
- (2010) Nigerian National Guidelines on Pediatric HIV/AIDs Treatment and Care.
- Seema TM, Charlotte C, Beth C, Holly R, Oluwatoyin J, et al. (2014) Time-Dependent Predictors of Loss to Follow-Up in a Large HIV Treatment Cohort in Nigeria. Open Forum Infect Dis 1(2): ofu055.
- (2011) How to get to zero; faster, smarter, better. UNAIDS. Worlds AIDS Day Report.
-
Nwankwo Gloria Ogochukwu, Ogbonna Brian Onyebuchi, Anetoh Maureen Ugonwa, Ejieh Loveth, Adenola Ugochi Amanda, et al., Dynamics of The Universal Test and Treat Strategy in HIV Patients in Nigeria: A Narrative Review of Studies. On J Complement & Alt Med. 7(5): 2022. OJCAM.MS.ID.000671.
-
Universal test and treat strategy; HIV infection; Nigeria; Antiretroviral; HIV Patients; HIV treatment Guideline; Pregnant; Breastfeeding women; Adolescents; Diagnosis of HIV; HIV infection
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






