 Research Article
Research Article
Sustainable Keratin Fibre Generation: Dye Removal, Extraction, and Spinning of Wool Waste for Fashion and Textile Applications
Danmei Sun*, Milda Lebedytė and Nimra Nawaz
School of Textiles and Design, Heriot-Watt University, UK
Danmei Sun, Research wing, School of Textiles and Design, Heriot-Watt University, Galashiels, TD1 3HF, UK.
Received Date: January 15, 2025; Published Date: February 10, 2025
Abstract
This study investigates dye removal, wool dissolution, and keratin extraction from wool waste samples initially dyed with synthetic Bemacid E dyes. Dimethyl sulfoxide (DMSO) was selected for dye extraction due to its mild impact on dye structure; however, operational challenges led to the adoption of Decrolin (C2H6O6S2Zn), a reducing agent commonly used in textile bleaching. Decrolin’s dye removal was confirmed by UV-Vis analysis but showed potential re-oxidation effects over time, indicating incomplete dye extraction. Fourier Transform Infrared (FTIR) analysis revealed that while treatments affected sample coloration, keratin structures remained largely intact, although slight conformation changes were observed. The study examined wool dissolution using various reduction-based systems, finding that elevated temperatures (up to 80 °C) enhanced dissolution efficacy without significant keratin degradation. Dialysis and filtration further purified keratin solutions, producing variable concentrations dependent on treatment. SDS-PAGE analysis highlighted different molecular weights among keratin extracts, suggesting optimal extraction conditions for highmolecular- weight proteins, which are crucial for forming strong keratin filaments. A wet spinning process was used to fabricate keratin-polyvinyl alcohol (PVOH) filaments. Tensile testing showed that methylene diphenyl diisocyanate (MDI) cross-linking significantly enhanced filament strength, achieving a tenacity suitable for potential commercial application. The study concludes that while keratin fibres from wool waste display promising tenacity, further adjustments are needed to improve elongation, balancing strength and flexibility for industrial viability. Future research should refine extraction methods and optimize spinning parameters to enhance fibre properties.
Keywords: Wool waste; Keratin extraction; Fibre generation; Cross-linking; Chemical structure analysis; Physio-mechanical properties; New fibre feedstock
Introduction
Textile waste management has garnered increasing attention in recent years due to the environmental challenges posed by the textile industry, one of the most resource-intensive sectors globally [1]. Wool, a proteinaceous fibre primarily composed of keratin, represents a significant portion of textile waste. Keratin, a valuable biopolymer, holds potential for diverse applications, particularly in biomedicine, agriculture, and cosmetics. However, wool-based textile waste is often incinerated or sent to landfills, leading to environmental degradation. The innovative approach of generating keratin fibres from wool textile waste presents a sustainable solution to textile waste management, converting waste into valuable biomaterials.
Wool textile waste comprises post-consumer products, production scraps, and damaged or unwanted woolen garments. As natural fibres, wool products degrade slower than synthetic fibres but still contribute to landfill accumulation and resource depletion. Researchers have explored recycling methods, particularly in reusing keratin, due to wool’s high keratin content, which comprises about 90% of the fiber’s composition [2]. Transforming this waste into keratin-derived products addresses the environmental burden and introduces a value-added product to industries requiring biopolymers. Keratin is a fibrous structural protein rich in amino acids such as cysteine, which allows the formation of disulfide bonds, contributing to its stability and resilience. These properties make keratin suitable for applications like wound healing, tissue scaffolding, and cosmetic formulations [3]. The complex structure of keratin, particularly its cross-linked and helical arrangement, makes it more challenging to extract and process than other proteins.
Several methods have been developed to extract keratin from wool, including oxidative, reductive, and enzymatic techniques [4]. Oxidative and reductive treatments break down disulfide bonds, leading to keratin solubilization. Enzymatic methods, while milder, rely on specific proteases that cleave keratin structures without altering their functional properties, resulting in a more intact and usable biopolymer [5].
The transformation of wool waste into keratin involves several extraction techniques. Reductive Extraction is one of the most common methods that involves using reducing agents, such as thioglycolic acid or dithiothreitol, to break the disulfide bonds in keratin, rendering it soluble [6]. Reductive methods are effective but may require extensive purification to remove chemicals used during the process. Oxidative Extraction is a technique that employs oxidizing agents like hydrogen peroxide to break down wool fibres. However, oxidative methods can lead to keratin degradation and loss of functional properties [7]. Enzymatic Extraction that using Enzymes such as proteases to break down wool waste into smaller keratin molecules under mild conditions. Enzymatic extraction is more environmentally friendly but may result in lower yields than chemical methods [8]. In some cases, mechanical disruption is used in conjunction with other methods to assist in breaking down wool fibres, thereby increasing extraction efficiency [9].
Keratin extracted from wool waste has shown promise in several applications, but not in the textiles and fashion sectors. (a) Biomedical Applications: Keratin’s biocompatibility and biodegradability make it an attractive material for medical applications such as wound dressings, drug delivery systems, and tissue engineering scaffolds [10]. Keratin-based hydrogels and films have been explored for their ability to promote cell adhesion and proliferation, aiding in wound healing and tissue regeneration [11]. (b) Cosmetics: In the cosmetic industry, keratin is used for hair care products due to its restorative properties. Hydrolyzed keratin is often added to shampoos, conditioners, and hair treatments to improve hair strength and shine [12]. (c) Agriculture: Keratin waste has been proposed as a nitrogen-rich fertilizer in agriculture. The slow biodegradation of keratin provides a long-term nitrogen release, which can enhance soil quality and promote plant growth [13]. Figure 1 shows the forms of keratin in each step of the processes outlined thus far.

Converting wool textile waste into keratin biopolymers represents a sustainable approach to addressing textile waste. Traditional disposal methods, such as incineration and landfilling, contribute to greenhouse gas emissions and environmental contamination. The production of keratin from waste reduces environmental impact by extending the lifecycle of wool and lowering the demand for raw materials. Life cycle assessment (LCA) studies indicate that keratin extraction processes are generally less resource-intensive than the production of synthetic alternatives, making them a viable option for sustainable material development [14].
Despite the promising potential of keratin fibre generation from wool textile waste, several challenges remain. The efficiency of keratin extraction techniques varies and optimizing methods for large-scale production is crucial. Reductive and oxidative methods, while effective, often require harsh chemicals, raising concerns about environmental safety and the purity of the final product. Research is ongoing to develop greener extraction methods that balance efficiency and environmental sustainability [15]. Moreover, the application of keratin-based products in various industries is still in its early stages. More research is needed to refine the functional properties of keratin materials and tailor them to specific applications. Advancements in biopolymer engineering and nanotechnology may open new avenues for keratin-based innovations, further enhancing its value as a sustainable biomaterial.
Materials and Methods
Figure 2 shows a flow chart of processing wool waste.

Wool samples
Commercial wool fabric offcuts shown in Figure 3, were sourced from Schofield Dyers and Finishers (Galashiels, UK). These samples were dyed using Bemacid E dyes, a class of acid levelling dyes, along with various levelling agents and auxiliary chemicals during the dyeing process. Some lighter shades underwent mild bleaching to reduce yellowing, using Meropan Laso (stabilizer) and hydrogen peroxide (oxidizer). Additionally, all samples were finished with Glauber’s salt (levelling agent), Biavin BPA (lubricant), Kollasol CDS (antifoam), formic acid (pH adjuster), and CWL (levelling agent) at their facilities. Since the wool was decolorised and Soxhlet-scoured, these chemicals are not expected to affect the wool dissolution process.

Dye removal protocol
The original grey wool, which had already been Soxhlet scoured (6.07 g), was cut into small rectangles (1 x 2.5 cm) and refluxed with dimethyl sulfoxide (DMSO) (200 ml) for 30 minutes at 90 °C. The wool was then separated using gravity filtration, rinsed with distilled water, and left to dry in the back of the fume hood.
A yellow wool sample (8.7 g) underwent dye removal first, followed by Soxhlet scouring for 4.5 hours (instead of 24 hours), to assess whether the order of these processes would affect the wool.
As an alternative to the DMSO dye removal protocol, the original wool samples were treated with solutions of Decrolin® (also known by the registered trademarks Decroline, Decolin, and Safolin, Zinc formaldehyde sulfoxylate CAS 24887-06-7), a reducing agent used in the Textile Printing workshops at Heriot-Watt University. The wool samples were immersed in separate vials containing Decrolin/water solutions at concentrations of 1%, 4%, and 40% (Decrolin in excess) by weight/volume (w/v) for approximately 12 hours.
Soxhlet scouring protocol
Scouring wool removes any impurities left on the fibre. The wool was scoured via the Soxhlet apparatus with petroleum ether 40/60 for 24 h.
Wool dissolution
The availability of chemicals and equipment constrained the selection of dissolution methods for several types of mixed systems, as presented in Table 1.
Table 1: Types of systems tested, and the reagents present in each system, including notes on these systems selected.

The conditions for waste wool dissolution are as follows: Reducing solution with a pH range of 3 < pH < 14.5, temperatures below 55 °C, use of synthetic detergents such as sodium (n-)dodecyl sulfate (SDS) and reducing agents such as sodium sulfide. Table 2 lists the sample codes corresponding to each method type, which can be cross-referenced with raw data files and the lab notebook. Type I and III methods involve a chemical reduction process using sodium sulfide and SDS detergent; urea was used in W1 and W4. Type II methods also employed reduction, but also used sodium hydroxide (NaOH) (97%) instead of urea as an auxiliary chemical. Type IV method W9 used alcohol and a sulfitolysis reagent, sodium metabisulfite (Na2S2O5) (97%). Type V method W10 was adapted from Cao, et al. [16]. It employed a reduction method, such as Type I and II, but used a reducing agent of 1,4-dithiothreitol (DTT). The temperature used was at first under 50 °C as suggested by the Evans patent [89], but subsequently changed to 80 °C as suggested by Cao et al. This was later changed to 80 °C for methods: (1) Type III: W2, W3D13, W6D12, W12D14, W13 (and W13D15, W13D16) and W14; (2) Type IV method W9; and (3) Type V method W10. It seemed that the 80 °C method was more efficient, although the high temperatures could have led to some unwanted degradation of the keratin chains.
Table 2: Details of different methods and relevant sample codes.


Keratin filament spinning and cross-linking
Keratin was homogenously blended with PVOH (solution of 5 wt% in water) using different ratios (1:1 and 3:1). The solutions were extruded into a salt bath to achieve coagulation in the form of a filament. A diagram and a picture of the extrusion process used can be seen in Figure 4. A flat-end needle with an inner diameter of 1.4 mm (therefore approx. 17-gauge needle), 8.1 cm long, was used. The syringe pump (Harvard Apparatus, US, model 22) was adjusted to give an approx. 45° angle of the needle into the coagulation bath with the tip submerged. The solution was extruded at a rate of 0.01 ml/min into a 15 cm length coagulation bath containing ammonium sulphate ((NH4)2SO4) (30 wt% in water) adjusted to pH = 4 with hydrochloric acid. Once coagulation was seen at the syringe tip, a tweezer was used to slowly (manually) pull on the extruding solution and connect it to the makeshift wind-up roller, achieving a continuous filament. The wind-up roller was made of a bar inserted into the overhead stirrer, rotating at the slowest setting of approx. 30 rotations per minute.
After spinning, the filament was carefully removed and placed into the glutaraldehyde (GA) cross-linking solution (5 wt% GA in water) for 2 min, The fibre was then placed into a GA bath adjusted to pH = 4 with acetic acid for 2 min. Fibres were hung up to dry, then left in the vacuum oven overnight (Townson + Mercer, pressure: 0.65 bar at room temperature). The filaments were then crosslinked in a 4,4′-methylenebis- (phenyl isocyanate) (MDI) solution (2 wt% MDI in acetone) by submersion at 45 °C for 1 h. Some filaments were removed from the solution and dried at different parts of the process, as outlined in Table 3.
Table 3: Details of fibres formed by various spinning procedures.

Testing and analysis
FTIR analysis was carried out using the spectrometer Thermo Scientific Nicolet iS5, fitted with ATRID7/ITX AR Coated Diamond Crystal, with data visualization on Omnic software. FTIR analysis at different stages of the process was used as a rapid identification tool for the materials obtained.
Scanning Electron Microscopy (SEM) was used to observe the surface morphology of different solids under various processes. This included the original wool fabric, treated fabric, regenerated keratin, films, and filaments formed from polymers and keratin/ polymer blends. A Hitachi S-4300 (57E230) Version 09-03-0922 (PC), 09-02 (SEM) was used at an operating voltage of 1 kV. There was only minimal static charge build-up in some samples, but this was not so strong that spraying with metal ions was required.
Sodium n-Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) analysis was carried out at the Heriot-Watt G24 Laboratory. Figure 5 shows the experimental set-up. Sample solution (50 μL) was mixed with Sample Buffer (Laemmli 2x Concentrate, Sigma Aldrich), centrifuged (1 min at 2000 rpm), and then heated (10 min at 70 °C). Aliquots (10 μL) were loaded into the wells of the prepared gel (Mini_PROTEAN TGX Precast Gels, Bio-Rad, cat. #456-1094), with one well containing the pre-stained standard (Blue Plus2 pre-stained Standard, Invitrogen) and one containing distilled water as a negative control. The gels were run using TGS buffer (10x Tris/Glycine/SDS, Bio-Rad) diluted in water (1:10) for 40 min at 180 V. The gel was then left on a shaker (overnight) in a container with the staining buffer containing aluminum sulphate hydrate (5% w/v, Fisher), ethanol (10% v/v, Sigma), Coomassie Brilliant Blue G250 (0.02% w/v, Sigma), orthophosphoric acid (8% v/v, Fisher) in distilled water. The gel was then treated for 24 h on a shaker with de-staining solution, containing ethanol (10% v/v), orthophosphoric acid (2% v/v) and distilled water. The gels were rinsed with distilled water before taking pictures.

Instron was used to test the keratin fibre/filament’s physical, mechanical properties. Figure 6 shows the machine setting, where the upper jaw moves up while the lower clip stays and the upper clip stops when the fibre/filament being tested breaks down. A low rate of extension 5 mm/min was used, and data was collected.

Results and Discussion
Dye removal
The wool waste samples used in this study were initially dyed with Bemacid E dyes. Various dye extraction methods can be employed depending on the desired stability of the extracted dye. DMSO (Figure 7) was selected for this study due to its mild conditions, which minimize changes to the dye’s chemical structure, particularly beneficial for sensitive natural dyes. In contrast, synthetic dyes, like those used here, are generally more resistant to degradation during extraction. A UV-Vis reading of the extracted dye solution confirmed successful dye extraction (Figure 8), though further analysis of dye structure for potential recycling applications was deemed outside the scope of this project and left for future research.


DMSO extraction, however, was not ideal. due to its requirement of a reflux system and its tendency to leave the fabric saturated with solvent. Therefore, Decrolin was adopted for its accessibility, ease, and safety. Decrolin contains zinc (II) formaldehyde sulfoxylate, a reducing agent commonly used in silk bleaching. Despite its effectiveness, formaldehyde production—known to be carcinogenic, raises safety concerns, particularly for industrial scaling, and should be addressed.
Both DMSO and Decrolin discoloured the wool samples similarly, although subsequent infrared (IR) analysis suggested that some dye may have re-oxidized over time, restoring the fabric’s original colour. The re-oxidation likely occurred due to incomplete dye removal, a known issue with reducing agents used for bleaching.
FTIR analysis of Soxhlet scouring
Fourier transform infrared (FTIR) analysis of the Soxhlet- scoured samples (Figure 9) showed the expected keratin amide peaks. The Amide A peak is largely masked by the -OH peak, due to the sample being wet, but is probably at ~ 3272 cm-1, within the expected range of ~ 3286 cm-1. Amide B is probably not very visible because this wool is dyed, so the peak is of low intensity; it is expected to be in the range 3056 – 3075 cm-1. Amide A and B are caused by N-H stretching vibrations. Peaks at 1629, 1513 and 1231 cm-1 correspond to amides I, II and III respectively. Amide I is caused by C=O stretching, amide II by a mixture of N-H bending and C-N stretching, and amide III by a (phase) combination of C–N stretching and N–H bending vibrations. The broad OH peak shows that the sample was still wet when testing in Decrolin-treated and scoured fabrics and is in the same range as the A and B amide peaks. There are no distinctive differences in peak placements between the different traces, suggesting that treatments did not affect the keratin structure, as expected. However, this could also be interpreted as the wool not being decolorized and scoured sufficiently, as by the end, it looks identical to the original (dyed) wool. It could be argued that this is visible to some extent by the variability and change in intensity of the peaks, as this suggests changes in keratin molecule conformation. However, no significant differences were observed between the spectra of dyed, decolorized, and scoured wool, suggesting the treatments did not substantially affect the keratin structure. The intensity variation in the peaks might indicate changes in keratin conformation, though the samples were likely too wet to yield fully accurate results.

Dissolving wool

Chemical availability and lab equipment restricted the choice of dissolution methods to several reduction-based systems. Although lower temperatures (~50 °C) were initially tested to prevent keratin chain degradation, stirring at 80°C proved far more effective without significantly impacting chain length, as confirmed by SDS analysis. This finding highlights the importance of temperature in wool dissolution, with SEM analysis for further underscoring its impact.
Additionally, steam treatment using sodium hydroxide dissolved wool from mixed fabric samples, leaving the cotton intact, presented in Figure 10. However, the resulting keratin material adhered to glassware, limiting its use in subsequent regeneration and analysis steps. While promising, this method requires further refinement for practical application in future studies.
The most successful wool dissolution methods involved simpler systems (Type I and III), which relied on readily available reagents. These methods dissolved wool efficiently and were easier to apply in laboratory conditions. Conversely, Type II methods worked only partially due to time constraints, while Type IV (alcohol and sodium metabisulfite) and Type V (using DTT) failed to dissolve the wool under the conditions tested. The failure of Type IV may be attributed to keratin chain rearrangement, which inhibits the accessibility of disulfide bonds for reduction, while Type V likely suffered from DTT oxidation during prolonged stirring in an unsealed system. These methods should be re-examined in future studies under more controlled conditions.
Filtration and dialysis
After wool dissolution, filtration and dialysis were used to remove undissolved material and purify the keratin solution. Various treatments produced solutions with varying keratin concentrations, shown in Table 4. While results varied, the keratin concentration from method W4 (11.34 wt%) indicated a ~75% conversion rate. However, the concentrations for W13 and W14 after mesh filtration exceeded the theoretical yield of 15 wt%, suggesting that unreacted reagents or detergent residues may have contributed to the weight. Dialysis with 12-14 kDa and 6-8 kDa MWCO tubing yielded keratin concentrations of 18% and 34% of the filtered solution, respectively, indicating a significant amount of keratin was lost in the higher MW range. Future studies should use MWCO tubing below 12 kDa to retain low-molecular-weight keratins while removing reagents.
Table 4: Concentration in weight % (wt%) and g/ml of different solutions after different treatments.

SDS-PAGE analysis
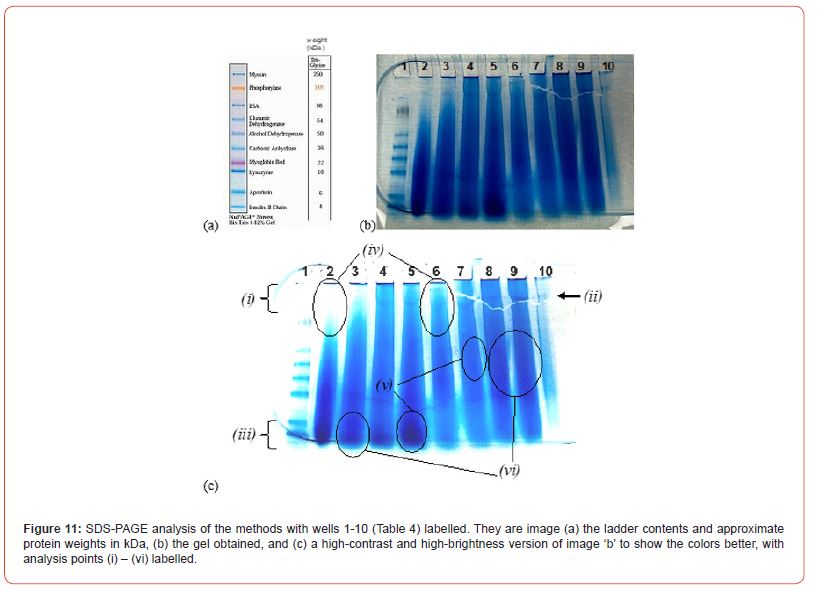
The molecular weight (MW) of keratin is crucial for forming effective filaments. Various extraction methods were analysed via SDS-PAGE. The well contents in the gels, presented in Table 5, highlight the different extraction methods and their resulting protein profiles, including various keratin extracts and a ladder. Figure 11 provides a visual representation of the SDS-PAGE analysis, with images highlighting different protein weights in kilodaltons (kDa), the gel results, and a high-contrast version to enhance band visibility. The key analysis points (i) to (vi) are annotated.
Table 5: Well contents in SDS-PAGE analysis. More information can be found in Appendix A1.
The distinct bands observed indicate a specific chain length of keratin rather than a broad MW distribution. Importantly, the absence of protein fragments below 10 kDa suggests that the primary protein chains remained largely intact. Literature supports the impact of reducing agents and reaction conditions on such results [15].
Samples in wells 2-4 contained predominantly low-MW proteins, evidenced by dark bands at the bottom of the gel. In contrast, wells 6-9 samples exhibited fewer low-MW proteins, with darker regions in the MW range of 64-16 kDa. This is critical because high-MW proteins contribute to stronger films and filaments, as their longer keratin chains offer enhanced structural stability. The low-MW keratin fragments were likely removed during dialysis using 12-14 kDa MWCO tubing. However, the general lack of distinct bands suggests potential primary chain degradation, possibly due to elevated temperatures during extraction. Despite using 12-14 kDa MWCO tubing, bands below 12 kDa were present, indicating incomplete dialysis. Analysis of wells 2-9 showed variations in high-MW keratin concentrations, with well 9 containing the highest amount, followed by wells 8, 4, and 5, while well 2 had minimal high-MW keratin. The presence of keratin below 12 kDa in some wells (e.g., Figure 9 c, (iii)) may reflect incomplete dialysis, which could affect future extraction trials. The SDS-PAGE analysis also allowed for some comparison between the extraction methods:
• Type I Methods: Wells 2, 4, and 6 utilized high urea concentrations and varying temperatures. Well 2, with the lowest temperature (~30 °C), showed significant low-MW keratin extraction, likely due to the urea-induced chain degradation. Well 6, which used higher temperatures (80 °C) and longer stirring (24h), surprisingly had less low-MW keratin, indicating that elevated temperature alone does not significantly increase chain degradation (Figure 9 c, (iv)).
• Type II Methods: Wells 5 and 7, using similar reagent concentrations but different temperatures (50 °C in well 5 and 80 °C in well 7), showed higher MW keratins in well 7. This suggests that temperature does not contribute to significant keratin degradation, as confirmed by the band patterns in the gel (Figure 9 c, (v)).
• Type III Methods: Wells 3 and 8 used the same conditions, with well 9 representing a more concentrated version of well 8. As expected, wells 8 and 9 showed similar high-MW keratin profiles, whereas well 3, which used double the concentration of reagents, showed more degradation with fewer high-MW proteins (Figure 9 c, (vi)). This suggests that higher concentrations of SDS and reducing agents lead to increased keratin chain fragmentation.
Characteristics of keratin and filament
FTIR analysis of acetone-regenerated and a cast extraction solution (Figure 12) showed that most of the same keratin peaks are present as seen in the fabric before and after treatment. The blue arrows show the keratin’s distinctive peaks, as previously described in Figure 9.
Both the regenerated sample and the cast sample had medium peaks at ~ 2900 cm-1, caused by C-H stretching. These peaks are not just absent from other FTIR spectra (wool fabric, decolorized and scoured, previously seen in Figure) but are more prominent in the concentrated directly cast sample (Figure 12 b)) than the regenerated sample (Figure 12 a)). Both keratin types (conformations) should be the same (β-keratin), so this difference is probably caused by interference between remaining chemicals, water or impurities present in the solution. It is possible that these materials get caught in the keratin structure during casting, as opposed to regenerated keratin, which is in a purer form of the material. This can also explain the peak seen at 1053 cm-1, often attributed to S=O stretching (sulfoxide from thiosulfate ion), usually attributed to cysteine- S-sulphonate (sometimes called ‘Bunte salts’) formed during extraction. The peak at 979 cm-1 is caused by the glycine-alanine periodic sequence in the structure, which is probably present as it has been seen for natural keratin (in silk) [17]. This could be more visible in Figure 10 b) due to extra heating when concentrating the solution, and any impurities present in the structure that have become trapped during casting, which have therefore interfered with some of the bond vibrations.

SEM analysis of regenerated materials showed that methods that did not stir at high enough temperature and long enough, W4 and W5, did not degrade the wool (Figure 13 c-d). In fact, these methods barely affected the cuticle scale structure of the original fibre (Figure 14 a). This is compared to the fact that no wool structures were seen in several samples stirred for longer, at higher temperatures, which were also mesh filtered and centrifuged to remove undissolved materials prior to dialysis, such as methods W4D4 and W2D11 (Figure 13 e-f). Figure 13 (f) shows some craters, probably from bubbles trapped in the structure.

The keratin solution was further processed for keratin fibre through a wet spinning process using a syringe as a spinneret in a cylindrical shape with a diameter of 1.4mm (approx. 17 gauge). The strength of the filaments was tested using Instron. Figure 14 shows the results from the tensile test, where the fibre with MDI cross-linking shows a much higher tenacity under the same elongation compared to the filament without a cross-linker.
Regarding the tenacity of the Gb/IIb traces, it is evident that adding MDI increased the maximum tenacity by 57% while reducing the elongation at the break by approximately 14%. For Hb/IIIb, the maximum tenacity values of both traces are very similar (0.763 and 0.774 cN/tex, respectively), though the elongation at break increased by an amount comparable to that seen in Gb/IIb with MDI addition. Increased cross-linking is generally expected to enhance a material’s tenacity. It is suggested that a tenacity of around 5 g/den (0.5448 cN/tex) is required for a fibre to be commercially viable. In these cases, the tenacity at break exceeds this threshold. Compared to other fibres in the textile industry, these fibres demonstrate tenacity in the upper range, though the elongation at break remains relatively low. Further improvements in elongation are needed while maintaining high fibre strength.

Conclusions and Implications
This study effectively optimized keratin extraction and filament formation from wool, with Decrolin replacing DMSO for safer, practical discoloration, though minor dye remnants persisted. Wool dissolution was achieved efficiently at higher temperatures (~80 °C) in simpler systems, avoiding significant keratin degradation. Filtration and dialysis techniques varied in effectiveness, with low- MWCO tubing suggested for improved keratin fragment retention.
FTIR analysis confirmed that keratin’s structure remained largely intact, though minor conformational shifts were seen, possibly due to incomplete decolorization. SDS-PAGE highlighted a distribution of keratin molecular weights, with higher-MW fractions forming stronger films. SEM analysis revealed that prolonged processing at higher temperatures disrupted wool structure, while lower-temperature methods retained the original fibre.
MDI cross-linking enhanced filament strength by 57% in Gb/ IIb samples, though elongation slightly decreased. High tenacity, reaching commercial viability standards, was achieved; however, improved elongation remains a future goal for maximizing both strength and flexibility.
Limitations
1. Dye Removal. Some re-oxidation of dye molecules was observed, indicating that the reducing agents used for bleaching may not completely degrade dye molecules, affecting the final purity of the wool.
2. Residual Solvent. The use of DMSO and Decrolin left residues in the wool matrix, which may impact downstream applications.
3. Keratin Chain Degradation. Variability in SDS-PAGE band intensity suggests potential degradation of keratin chains during dissolution, which could affect its mechanical properties in regenerated materials.
4. Processing Constraints. The requirement for controlled laboratory conditions, such as reflux systems and dialysis, may limit the scalability of these methods in industrial settings.
Recommendations for Future Research
1. Alternative Dye Removal Methods. Investigating more environmentally friendly and efficient dye extraction techniques, such as enzymatic treatments, to minimize chemical waste and health hazards.
2. Optimization of Dissolution Conditions. Refining temperature, reagent concentration, and reaction time to minimize keratin degradation while maintaining high extraction yields.
3. Enhanced Filtration Techniques: Investigating improved filtration and dialysis techniques to maximize keratin purity and reduce reagent contamination.
Some Potential End-Use Applications of The Regenerated Wool Fibre
1. Fashion and Textile Industry. Regenerated keratin fibres can be used in sustainable textiles, offering biodegradable alternatives for performance fabrics and nonwoven applications.
2. Biomedical Applications. Purified keratin holds potential for wound dressings, tissue scaffolding, and drug delivery systems due to its biocompatibility and structural properties.
3. Filtration and Adsorption Materials. Processed keratin materials can be explored for water purification and air filtration due to their ability to bind heavy metals and pollutants.
4. Sustainable Composites. Wool-derived keratin can be integrated into bio-based composites for automotive and construction applications, reducing reliance on petroleum-based polymers.
Overall, this study highlights the potential of sustainable wool processing and keratin extraction for various industries while identifying key challenges that future research should address. By refining these processes, wool waste can be effectively repurposed, contributing to circular economy initiatives and reducing environmental impact.
Acknowledgement
None.
Conflict of Interest
Authors declare no conflict of interest.
References
- Sandin G, Peters GM (2018) Environmental impact of textile reuse and recycling – A review. Journal of Cleaner Production 184: 353-365.
- Rajabinejad H, Peikari M, Sadeghi M (2020) Sustainable approaches to wool waste: Keratin fiber extraction and application. Waste and Biomass Valorization 11(5): 1453-1466.
- Shavandi A, Silva TH, Bekhit AA, Bekhit AE.-DA (2017) Keratin: Dissolution, extraction, and biomedical application. Biotechnology Advances 35(2): 251-265.
- Rouse JG, Van Dyke ME (2010) A review of keratin-based biomaterials for biomedical applications. Biomaterials 31(4): 319-329.
- Schrooyen PMM, Dijkstra PJ, Oberthür RC, Bantjes A, Feijen J (2001) Stabilization of solutions of feather keratins by sodium dodecyl sulfate. J Colloid Interface Sci 240(1): 30-39.
- Gupta A, Sharma S (2017) Extraction of keratin protein from chicken feathers using various methods and its potential applications. Waste and Biomass Valorization 8(1): 1-7.
- Sharma S, Gupta A, Thind SS (2018) Extraction of keratin from natural resources and its applications: A review. International Journal of Biological Macromolecules 108: 674-686.
- Tesfaye T, Sithole B, Ramjugernath D, Valorization T (2017) Valorisation of waste chicken feathers: Optimisation of keratin extraction conditions through response surface methodology. Waste and Biomass Valorization 8(4): 1043-1052.
- Ganesan P, Chauhan N, Muralidharan C (2018) Efficient extraction of keratin from wool waste using a combined physical and chemical method. Journal of Cleaner Production 198: 1192-1200.
- Shavandi A, Silva TH, Bekhit A El-Din A, Bekhit AA (2017) Keratin: Dissolution, extraction and biomedical application. Biomaterials Science 5(9): 1699-1735.
- Sierpinski P, Garrett J, Ma J, Apel PJ, Klorig DC, et al. (2008) The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 29(1): 118-128.
- Gniadecka M, Nielsen OF, Wessel S, Wulf HC (2018) Hydrolyzed keratin in hair care: Effects on hair structure and strength. Journal of Cosmetic Science 69(5): 303-312.
- Gupta A, Srivastava JK (2014) Utilization of keratin waste as organic nitrogen fertilizer for plant growth. Journal of Cleaner Production 81: 47-51.
- Ghebus LM (2022) Life Cycle Assessment of Keratin Extraction from Organic Waste. Master’s Thesis, Politecnico di Milano, School of Industrial and Information Engineering.
- Mouro C, Martins R, Gomes AP, Gouveia IC (2023) Upcycling Wool Waste into Keratin Gel-Based Nanofibers Using Deep Eutectic Solvents. Gels 9(8): 661.
- Cao GS, Ron, MZ, Zhang MQ (2020) Continuous High-Content Keratin Fibers with Balanced Properties Derived from Wool Waste. Acs Sustain Chem Eng 8(49): 18148-18156.
- Zoccola M, Aluigi A, Vineis C, Tonin C, Ferrero F, et al. (2008) Study on Cast Membranes and Electrospun Nanofibers Made from Keratin/Fibroin Blends. Biomacromolecules 9(10): 2819-2825.
-
Danmei Sun*, Milda Lebedytė and Nimra Nawaz. Sustainable Keratin Fibre Generation: Dye Removal, Extraction, and Spinning of Wool Waste for Fashion and Textile Applications. J Textile Sci & Fashion Tech 11(2): 2025. JTSFT.MS.ID.000760.
-
Wool waste, Keratin extraction, Fibre generation, Cross-linking, Chemical structure analysis, Physio-mechanical properties, New fibre feedstock
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






