 Research Article
Research Article
Preparation of Activated Carbon Fibers from Fiber Mixtures
Tsuyoshi Yoda1,2*, Keita Shibuya3 and Hideki Myoubudani3
1Chuetsu Technical Support Center, Industrial Research Institute of Niigata Prefecture, Japan
2Hirosaki Industrial Research Institute, Aomori Prefectural Industrial Technology Research Center, Japan
3Material Applied Technical Assistance Center, Industrial Research Institute of Niigata Prefecture, Japan
Tsuyoshi Yoda, Hirosaki Industrial Research Institute, Aomori Prefectural Industrial Technology Research Center, 1-1-8 Ougi-machi Hirosaki city, Aomori 036-8104, Japan.
Received Date: September 05, 2018; Published Date: September 14, 2018
Abstract
Activated carbon fiber (ACF) is a novel material that is attracting significant attention. The pore structure found on the surface of ACFs is strongly related to their functionality. ACF production from fiber mixtures is considered to be a useful approach for reusing waste cloth and fibers. However, the use of such mixtures of materials in the preparation of ACF has not been investigated in detail. Here, we describe ACF preparation from fiber mixtures and discuss solutions to a problem encountered during preparation.
Keywords: Activated carbon fiber; Fiber mixture; Thermal treatment; Adsorption function
Introduction
Activated carbon fiber (ACF) is a novel material. It has been increasingly attracting significant attention as a research area [1- 4], particularly because the pore structure on the surface of ACF likely has the potential to adsorb and retain undesired substances. Consequently, ACF is considered to be a potential alternative to activated carbon (eg; Activated charcoal made from waste palm shell, ACP), which has conventionally been used as a filter to remove waste products. Thus, effective methods for the production of ACF have been investigated [4,5]. Some methods have focused on exploring chemical modification of the pores on the ACF surface [5]. Recently, the activation of several different types of carbon sources has also been evaluated. At present, preparation of ACF from agricultural wastes such as banana peels [5] and almond shells [6] is of interest.
In most studies, ACF has only been prepared from one material such as silk [2], banana peels [5], almond shells [6], cow dung [7], peach stones [8], and cotton [9]. However, ACF production from a mixture of fibers is expected to be an ecologically friendly approach to reusing waste cloth and fiber materials. The use of mixtures of such materials for the preparation of ACF has not been fully investigated. Here, we describe ACF preparation from fiber mixtures such as those that may be a waste by-product of clothing production.
A tubular furnace, a simple instrument for thermal treatment that has been previously employed for the production of activated carbon, was used for the production of ACF in this study. Our results highlighted an issue in the preparation of ACF from fiber mixtures. To counter this, improvements in the production methods are also discussed.
Materials and Methods
Fiber material
A fiber mixture (JIS test fabric no. 0803, containing polyester 22.6%, viscose-rayon 12.8%, worsted 12.8%, cotton 11.3%, acrylic 11.3%, silk 10.4%, diacetate 9.8%, and nylon 9%) was purchased from the Japanese Standards Association. We considered that this mixture would serve as a model for cloth waste.
Thermal treatment
Samples were carbonized at various temperatures in a tubular furnace (ISUZU, KRO-14) with a temperature control unit (CHINO, MODEL-SU) under a flow of nitrogen or carbon dioxide gas.
Adsorption of methylene blue
To determine the adsorption of methylene blue (MB) onto the ACF materials, we referred to test methods for activated carbon given in Japan Industrial Standard K-1474 [10]. Briefly, we began with a 1200 mg/L aqueous MB solution that was diluted to concentrations of 120, 24, 12, 2.4, 1.2, 0.24, and 0.12 mg/L using a phosphate buffer made from potassium dihydrogen phosphate, disodium hydrogen phosphate, and Milli-Q pure water. The optical absorbance of these solutions was measured using a spectrophotometer (U-3210, HITACHI, Japan) to obtain a calibration curve. We then measured the absorbance of the 120 mg/L MB solution after adding 1 g of ACF, or ACP as a positive control, and shaking for 30 min. We calculated the level of adsorption as the amount of MB (mg) adsorbed by the activated carbon (ACF or ACP, g) based on the optical absorbance of the solution and the obtained calibration curve.
Sample labeling
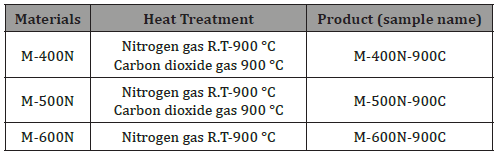
The sample treatments were as follows: untreated fiber mixture (M), fiber mixture heat-treated under carbon dioxide at 900 °C (M-900C), fiber mixture heat-treated under nitrogen at 400 °C (M-400N), fiber mixture heat-treated under nitrogen at 500 °C (M-500N), and fiber mixture heat-treated under nitrogen at 600 °C (M-600N) (Table 1). Samples of the M-400N, M-500N, and M-600N treatments were also subjected to a second heat treatment under carbon dioxide at 900 °C and are termed as M-400N-900C, M-500N- 900C, and M-600N-900C, respectively (Table 2).
Table 1: Summary of heat treatment processes for carbonization of fibers. Each process and sample name are listed.
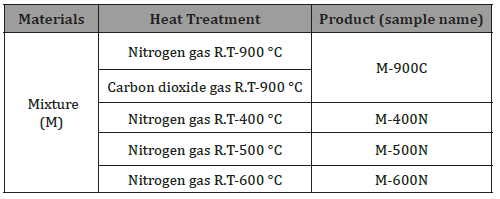
Table 2: Summary of heat treatment processes for activated carbon fibers. Each process and sample name are listed.
Results and Discussion
Sample yields
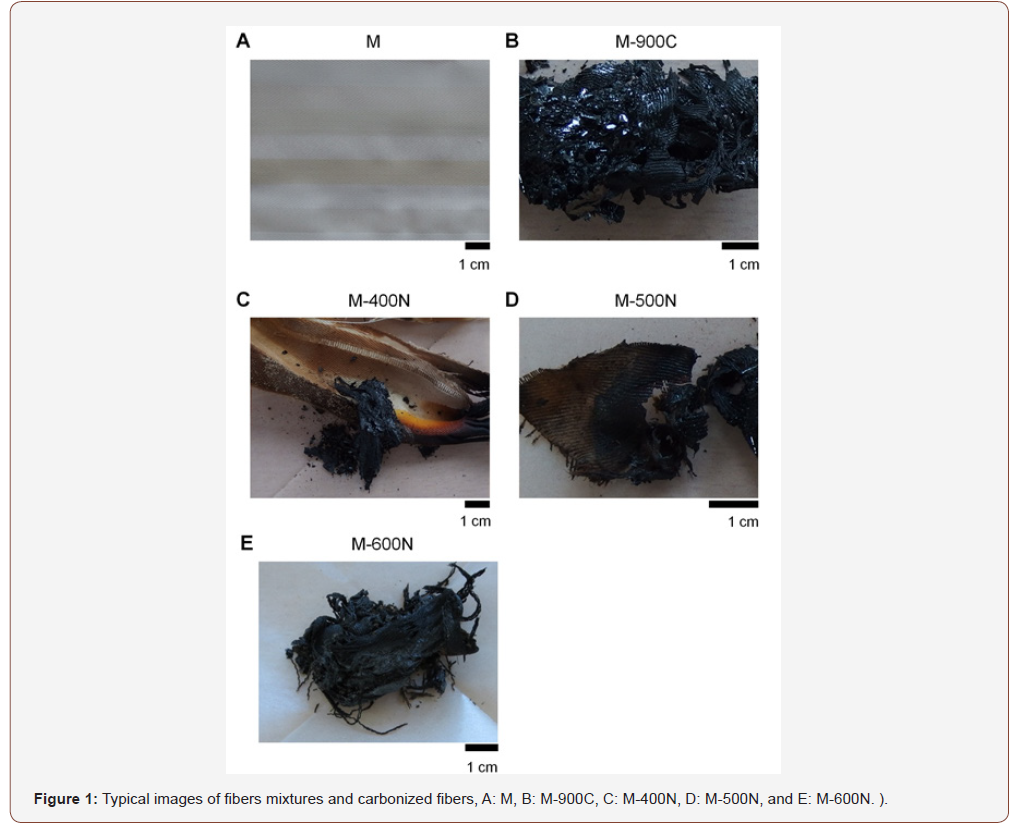
Typical photos of the untreated and treated fiber mixture samples are shown in Fig. 1. The heat-treated samples were fragile, and hence, images were obtained in the tubular state (Figures 1A-1E). The heat-treated samples under carbon dioxide were particularly fragile and their images were also obtained in the tubular state (Figures 2A-2C).

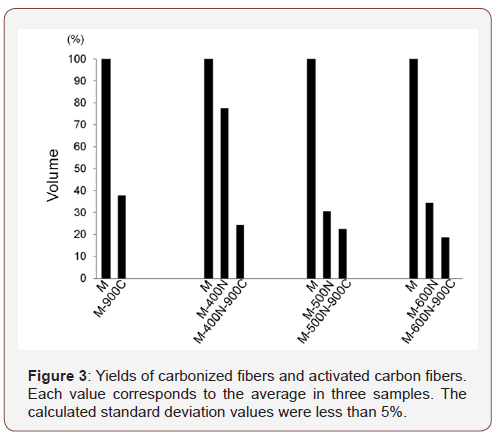
The samples yield, which were calculated using the method of Martinez et al. [8], were 37.5%, 77.4%, 24.3%, 30.0%, 22.2%, 36.4%, and 18.6% for M-900C, M-400N, M-400N-900C, M-500N, M-500N-900C, M-600N, and M-600N-900C, respectively (Figure 3). Yields for activated carbon from cotton [9], walnut shells, and peach stones [8] have been reported to be 37.92%, 20.8%, and 22.2%, respectively. Although the yields obtained in the present study were not an improvement over previously published results [11], the yields of M-900C and M-600N were similar to published values (Figure 3).
Adsorption
MB was chosen for this study because it is known to strongly adsorb on to activated carbon materials [10]. Typical MB adsorption isotherms obtained for ACP, M, M-900C, M400N-900C, M500N-900C, and M600N-900C are shown in Figure 4. Compared with ACP, adsorption was lower for all samples derived from fiber mixtures, although the heat-treated mixtures did exhibit greater MB adsorption than the untreated fiber mixture. MB adsorption values of ACFs derived from fiber mixtures using the two-step thermal treatment were all smaller than those obtained for the onestep preparation (M-900C, Figure 4). Although we previously found that the two-step thermal treatment is efficient for cotton [12], this study finds that the two-step thermal treatment is not an effective method for the preparation of ACFs derived from fiber mixtures. These results indicate that one-step thermal treatment may be a better approach for ACF production using fiber mixtures. However, the adsorption ability was not dramatically improved by thermal treatment when compared with cotton and rayon.
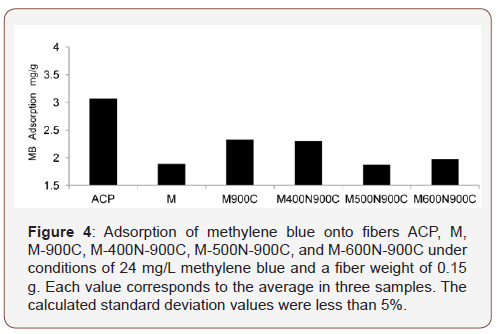
Our results indicate that the adsorption ability of ACFs derived from fiber mixtures is not effectively increased. The melting point of polyester is 260 °C [13], which is lower than the temperature conditions used in the present study. Therefore, polyester should have liquefied during thermal treatment, resulting in the polyester fibers closing the pore structure generated from cotton, rayon, and other fibers. In this scenario, the pore structure generated from non-polyester fibers would have a lower propensity for adsorption. Adsorption of ACFs produced using the one-step thermal treatment showed greater MB adsorption than that of the two-step thermal treatment. This suggests that the pore structure of other carbonized fibers became closed due to repeated heating in the presence of polyester.
Recently, our group reported that preparing ACFs from mixtures of cotton and polyester fibers, characterizing the products, and measuring their adsorption abilities [14]. The study indicated that results of the MB adsorption experiments clarified that treatment at 600 °C under carbon dioxide produced ACF made from cotton and polyester mixtures with good adsorption ability. These previous results and agreed with results of our present study. Untreated polyester films have adsorption abilities [14,15]. To improve adsorption ability when using filter mixtures, it is suggested that polyester should be removed from mixtures of pore-generating fibers, such as cotton and rayon, prior to thermal treatment. Our research findings will be useful in recycling cloth waste into heattreated ACF products that can be used to trap and retain undesired substances.
Conclusion
We reported on the preparation of ACFs derived from fiber mixtures using two different thermal treatment processes and characterized the products and their adsorption abilities. The results of MB adsorption experiments revealed that the two-step thermal treatment is not effective for the preparation of ACF derived from fiber mixtures that include polyester. These results may be useful for better understanding the adsorption abilities of ACFs derived from mixed fiber wastes that include fibers with a low melting point, such as polyester.
Acknowledgement
We would like to appreciate Dr. Kohei Isobe partially for his help in English editing of the manuscript and kindly suggestion.
Conflict of Interest
Author said no Conflict of Interest.
References
- Myoubudani H, Watanabe R, Kasahara K, Okada H (2014) Report of the Industrial Research Institute of NIIGATA Prefecture. 43: 85.
- Yoda T, Shibuya K, Miura K, Myoubudani H (2017) Measurement. 101: 103.
- Myoubudani H, Shibuya K, Kasahara H, Okada H, Miura K, et al. (2015) Report of the Industrial Research Institute of NIIGATA Prefecture. 44: 71.
- Pandolfo AG, Hollenkamp AF (2006) Carbon properties and their role in supercapacitors. 157(1): 11-27.
- Sugumaran P, Susan VP, Packiyam R, Seshadri S (2012) Production and characterization of activated carbon from banana empty fruit bunch and delonix regia fruit pod. J Sustainable Energy Environ 3: 125-131.
- Pragya P, Sripa S, Kumar YM (2013) Preparation and study of properties of activated carbon produced from agricultural and industrial waste shells. Res J Chem Sci 3(12): 12-15.
- Demiral H, Demiral I (2008) Surface properties of activated carbon prepared from wastes. Surf. Interface Anal 40: 612-615.
- Martinez ML, Moiraghi L, Agnese M, Guzman C (2003) Making and some properties of activated carbon produced from agricultural industrial residues from Argentina. J Argent Biochem Soc 91: 103-108.
- Uddin MJ, Cesano F, Bonino F, Bordiga S, Spoto G, et al. (2007) Photoactive TiO2 films on cellulose fibres: synthesis and characterization. J Photochem Photobiol 189(2): 286-294.
- Japanese Industrial Standards (JIS) (2014) Test methods for activated carbon. K-1474.
- Sun J, Satyapal S (1999) Methanol adsorption on activated carbons for adsorption heat pump applications. 652-653.
- Yoda T, Shibuya K, Miura K, Myoubudani H, submitted to International journal of Materials and Structure integrity.
- Speight J (2005) Lange’s Handbook of Chemistry. (16th edn), McGraw- Hill Education, Newyork, USA.
- Yoda T, Shibuya K, Myoubudani H (2018) Measurement. 128: 572-576.
- Giles CH, Hojiwala BJ, Shah CD (1972) Quantum efficiency measurements of fading of some disperse dyes in nylon and polyester films and in solution. JSDC 88: 403-407.
-
Tsuyoshi Yoda. Preparation of Activated Carbon Fibers from Fiber Mixtures. J Textile Sci & Fashion Tech. 1(2): 2018. JTSFT.MS.ID.000510.
-
Activated carbon fibers, Novel material, Cloth, Fiber mixture, Thermal treatment, Adsorption function, Silk, Tubular furnace, Polyester, Methylene blue, Spectrophotometer, Adsorption, Rayon, Carbonized fibers, Recycling
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






