 Research Article
Research Article
Feasibility Analysis of the Implementation of Ecofriendly Biological Pretreatment on Cotton Knit Fabric
Kazi Sirajul Islam1, Mohammed Farhad Mahmud Chowdhury2, Md. Sadman Sakib3, Nusrat Binta Hossain4, Md. Israt Bin Hossain5 and Mustafijur Rahman1*
1Department of Dyes and Chemical Engineering, Bangladesh University of Textiles, Bangladesh
2Department of Yarn Engineering, Bangladesh University of Textiles, Bangladesh
3Department of Industrial and Production Engineering, Bangladesh University of Textiles, Bangladesh
4Department of Environmental Science and Management, North South University, Bangladesh
5Department of Chemistry, University of Chittagong, Bangladesh
Mustafijur Rahman, Department of Dyes and Chemical Engineering, Bangladesh University of Textiles, Bangladesh.
Received Date: June 16, 2022; Published Date:August 01, 2022
Abstract
In this study an attempt was made to investigate bio pretreatment and chemical pretreatment process and observe the effects in terms of absorbency, bursting strength, wash and rubbing fastness, water, and energy consumption. Four samples (S1, S2, S3 and S4) were prepared by different pretreatment processes and then were dyed with two different reactive dyes. When compared to chemically prepared fabrics, biopretreated fabrics demonstrated improved abrasion resistance, bursting strength, and wash fastness. A significant improvement in sustainability aspects had observed in terms of lower demand for energy and water. This study focused to introduce an optimizable eco-friendly pretreatment process with enzyme application of the cotton knit fabrics.
Keywords: Scouring; Bleaching; Biopolishing; Dyeing; Fastness; Sustainability
Introduction
Knit fabric, a prevalent stretchable textile product, is very popular as apparel for its warmth, softer feel, comfort and wrinkle resistance it offers and so on. Moreover, knitted products made of cotton fibers can best supply these properties. Cotton fibers are mostly a-cellulose (88.0–96.5%) [1]. However, the specific chemical compositions of cotton fibers vary by their varieties, growing environments (soil, water, temperature, pest, etc.) and maturity. The noncellulosics include proteins (1.0-1.9%), waxes (0.4-1.2%), pectins (0.4-1.2%), inorganics (0.7-1.6%), and other (0.5-8.0%) substances. The waxes and pectins in cotton fibers are the noncellulosic components most responsible for the hydrophobicity or low water wettability of raw cotton fibers. [2].
The pretreating of cotton knitted fabric to improve its performance consists of three consecutive steps: scouring, bleaching and bio polishing. Scouring is the process of boiling textiles with alkaline agents, surfactants, or organic solvents. To remove the non-cellulosic impurities such as wax and pectin and improve the wettability, absorbency, and whiteness of textile materials [3,4]. The conventional chemical process consumes large quantities of NaOH and H2O2 (Conventionally, 7.3 % alkali and 6.9 % hydrogen peroxide [5]) and rinses water [6]. For instance, on average, The Indian textile industry requires 200–300 m3 of water for each ton of textiles that have been treated [6] that offloads environmentally harmful large amount amounts of wash effluent (salts, acids, and alkali) [7]. On the other hand, the bio scouring is followed by 5- 10% weight loss of the cotton [8], reduced tensile strength of the fabrics due to oxidative damage to cellulose, fabric shrinkage [9], High levels of energy, chemical oxygen demand (COD), biological oxygen demand (BOD5), and total organic carbon (TOC) in effluent water and necessitates special treatment of NaOH effluent [10].
Bleaching is designed to produce white goods by removing natural color matters carried out under alkaline conditions and must be accomplished with a minimum of damage to the cotton being bleached. The removal of these bleaching agents necessitates extensive washing and rinsing, which generates wastewater. The residuals hydrogen peroxide in the bath helps to decrease the COD by degrading the organic impurities in the effluent [10].
Biopolishing treatment using cellulases influences dyeability and physical properties of the fabrics such as strength loss, elongation gain, after treatments. In addition, it improves fabric appearance, moisture absorption and handles values that can otherwise be achieved using chemical finishes [11].
The textile industry is one of the industries that use the most water. A typical textile processing unit produces approximately 125L of effluent on average [12]. About 75% of the organic pollutants arising from textile finishing are derived from the preparation of cotton goods. After three cycles of reusing the scouring and bleaching bath, the effluent still contained 55% alkali and 67.50% hydrogen peroxide [5]. This chemical process must be significantly enhanced to address energy and environmental concerns. It is of utmost responsibility for minimal usage of dyes, auxiliary chemicals, water, and energy for minimal pollutant discharge from the textile industry. The strategies with the greatest potential for achieving these objectives are: (1) using alternative environmentally friendly auxiliary chemicals, (2) pretreatment of textiles to reduce the use of auxiliary chemicals, and (3) enzymatic textile processing to reduce auxiliary chemical use [13].
Bio-preparation may be a suitable substitute for the harsh chemical pretreatment process and with selected enzymes, it has the potential advantages over chemical pretreatment such as a higher quality of fibers, fewer wastewaters with lower BOD5, COD, and TOC, a shorter process time, cotton weight loss and harshness to handle, energy efficiency, and compatibility with other procedures, equipment, and materials. Enzymes are biologically degradable and risk-free to handle. Enzymes are proteins, i.e., sequences of amino acids linked by peptide bonds. Enzymes work by lowering the activation energy and the energy of the transition state for a reaction, thus speeding up the reaction rate magnificently [14,15].
Different individual enzymes and their mixtures—pectinases, cellulases, proteases, and lipases- were studied. [7,16-18]. Pectinases are the most suitable enzyme, capable of depolymerizing the pectin, breaking it down to low molecular water-soluble oligomers [19] and thereby improving the absorbency of the textile material without causing cellulose destruction.
According to life-cycle assessment (LCA) studies conducted by Novozymes, the use of enzymes in cotton textile production will reduce water consumption by up to 28%, chemical consumption by up to 80%, and energy consumption by up to 25% [20]. In the bleach clean-up, 1 kg of enzyme and in bio scouring, 10 kg of enzyme can save 20 m3 water per ton of yarn individually. In addition, in terms of global warming, the application of enzyme can save 1000 kg CO2 eq. in bio scouring and 420 kg CO2 eq. in the bleach clean-up [21]. It is reported that after 20 cycles of washings, Chemically scoured and bleached fabrics experienced greater loss of weight (3.27– 3.56%), lower loss of tenacity (16.8%) than the bio scoured and bleached fabrics as well as the bio scouring consumed 58.3% of the steam and 62.9% of the water over the chemical scoured process [10]. Laccase reduces peroxide consumption by approximately 50 percent [22]. BOD and COD of bio scouring are 20-45 %, and Total Dissolved Solids (TDS) of bio scouring are 20-50% as compared to alkaline scouring (100 %) [23].
Materials and Methods
Experiment design
Four types of samples were produced in the pretreatment stage. The designation of the different processes is defined in Table 1. Sample 1 (S1) was produced from single-stage conventional pre-treatment where chemical scouring was followed by chemical bleaching, bio peroxide killing and bio polishing, sample 2 (S2) was produced from single-stage biological pretreatment where bio scouring was(Extra Gap) followed by chemical bleaching, bio peroxide killing and bio polishing, sample 3 (S3) was produced from combined chemical pretreatment where combined chemical scouring & bleaching was followed by bio peroxide killing and in case of sample 4 (S4), combined biological pre-treatment was carried out where bio scouring was followed by conventional chemical bleaching and combined bio peroxide killing & bio polishing. These four pretreated samples were then dyed with two types of reactive dyes (Table 2).
Table 1:Process designation.
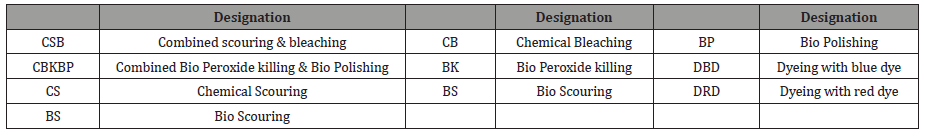
Table 2:Experiment design.
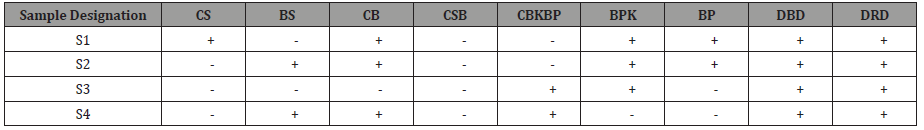
Materia
In this study, 100% cotton single jersey fabric, with a basis weight of 67.02 g/m2 and produced by Unitex group, Bangladesh was used. The chemicals used for this project are listed in Table 3.
Table 3:The Chemicals used in the experiment.
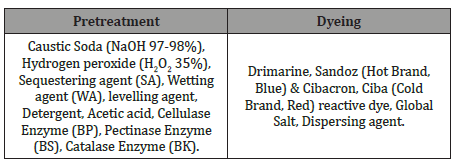
Treatment
Table 4 illustrates chemical proportion and Figure 1 states the process description. Scouring (CS) was performed with Caustic Soda (5 g.l-1) in an alkaline medium with M: L 1:50 at 90-100 °C. In contrast, bio scouring (BS) was carried out with pectinase enzyme (3 g.l-1) in an acidic medium with M: L 1:30 at a much lower temperature of 55-65 °C. Both the chemical bleaching (CB) and combined scouring and bleaching (CCSCB) was carried out with M: L 1:50 in alkaline medium with hydrogen peroxide (4 g.l- 1/,2 g.l-1), Caustic soda (3 g.l-1/5 g.l-1), and peroxide stabilizer (0.8 g. l-1/1 g.l-1) respectively at 90-100 °C. Moreover, wetting and sequestering agents were added to the bath. Bio peroxide killing (BK) with Peroxide killer enzyme Catalase (4 g.l-1) and bio polishing (BP) with enzyme Cellulase (2 g.l-1) both were performed with M: L 1:30 in alkaline medium at 50-60 ∘C. Combined bio peroxide killing & bio polishing (CBKBP) was carried out with enzyme Catalase (4 g.l-1), enzyme Cellulase (2 g.l-1) and acidic acid (2 g.l-1) in an acidic medium at 50-60 °C.
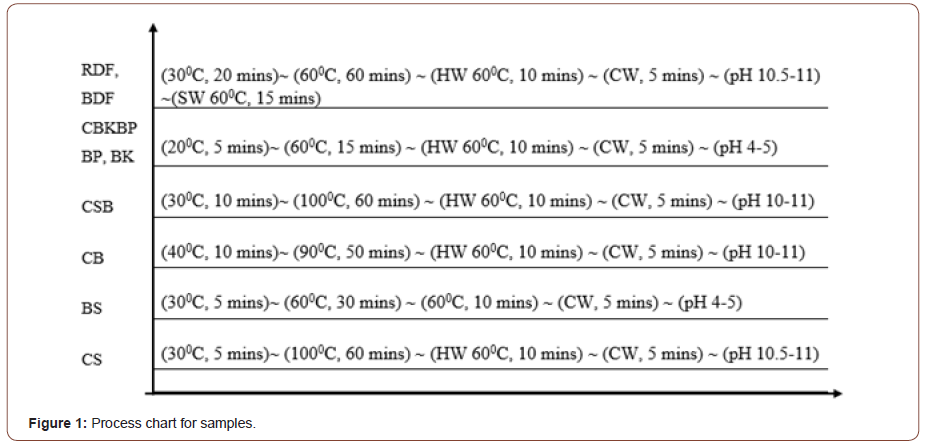
Table 4:Ingredient proportion in pretreatment process.
Dyeing
The reactive dye Drimarine with M: L 1:50 at 60 °C – 70 °C and Cibacron with M: L 1:40 at 50 °C – 60 °C were used (3%) with 1 % shade to dye the pretreated fabric. In the dye bath, 15 g. l−1 soda ash and 40 g. l−1 Glauber’s salt was added in an alkaline medium. Then soap wash was conducted with soda ash (0.5 g. l−1) and detergent (2 g. l−1).
Result and Discussion
Absorbency test
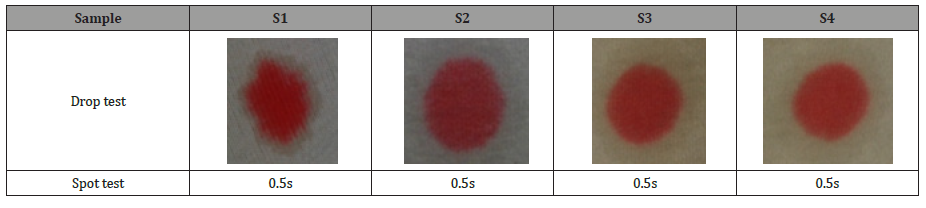
According to AATCC Test Method 79-2000, a drop of water is allowed to fall from a fixed height onto the taut surface of a test specimen during a spot and drop absorbency test. Wetting time is the measurement of the time required for the water drop’s reflection to disappear during the spot test. The wetting time of four samples was the same as 0.5s. The drop test measured the size and uniformity of the absorbed area using 0.1% direct dye solution. The size and uniformity of the absorbing area of bio-scoured fabric (S2) are better than other samples. Among these samples, the alkali scoured fabric (S1) was the worst in terms of size and uniformity. So, we can say that bio-scoured fabric is more hydrophilic than alkali scoured and fastest in absorbing liquor as well (Table 5).
Table 5:Result of the absorbency test.
Bursting strength
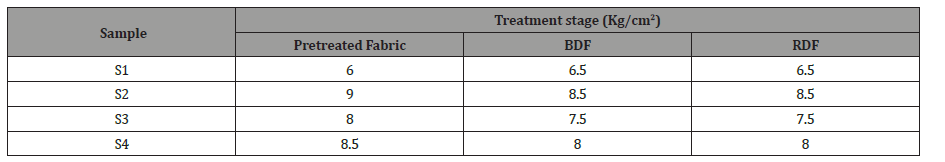
Table 6 represents the test result of the bursting strength of samples. The fabric bursting strength was tested using a JH Truburst tester machine according to the ISO 13938-2:2019 method (test area: 10 cm2, diameter: 35.7 mm).
Table 6:Result of the absorbency test.
Stage: Pretreatment
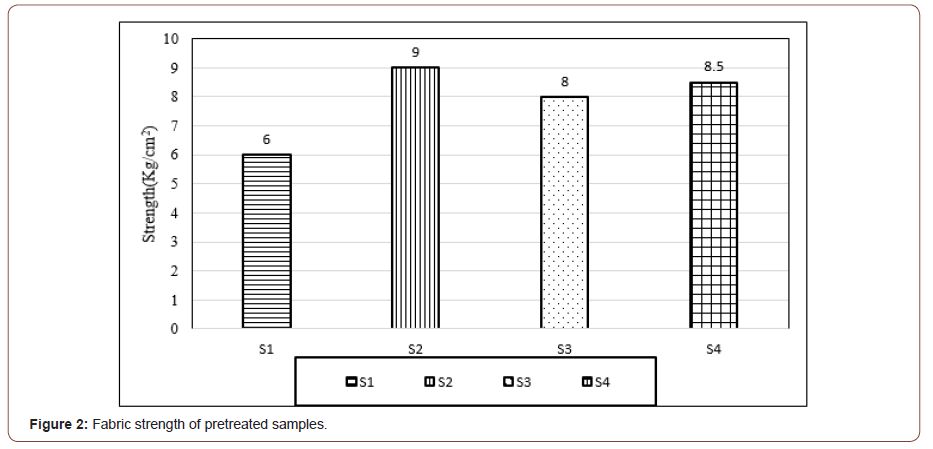
The bar diagram (Figure 2) illustrates the bursting strength test result of the four samples after pre-treatment process. The highest bursting strength (9 kg/cm2) was found in sample S2, produced from single-stage pre-treatment followed by sample S4, produced from a combined biological pre-treatment value (8.5 kg/cm2). It is noteworthy that in the case of sample S4, where a combined biological process was applied, strength dropped slightly more than sample S2.
On the other hand, the lowest strength has been observed from sample S1, produced from the single-stage conventional pretreatment (6 kg/cm2) though the bursting strength of sample S3, produced from combined chemical pre-treatment increased to 8 kg/cm2. This is visible that the value of bursting strength is higher in the biological pre-treatment process in comparison with other chemical pre-treatment process which consumes a significant amount of traditional chemicals. The tensile strength of the fibers mainly depends on the arrangement and capacity of the polymeric molecules to withstand the load.
Figure 3 represents the bursting strength of reactive dyed cotton knitted fabric. The four pretreatment samples were dyed with reactive dye (Blue dye, BDF & Red dye, RD). The diagram clearly shows that the highest strength was produced in case of sample S2 (single stage biological pre-treated fabric) and the value is 8.5 kg/cm2. The second highest strength (8 kg/cm2) has been observed for sample S4 (combined biological pre-treated fabric). In contrast, the lowest bursting strength (6.5 kg/cm2) has been found in the case of sample S1(single-stage chemical pre-treated fabric) followed by sample S3 (7.5 kg/ cm2), produced from combined chemical pretreatment.
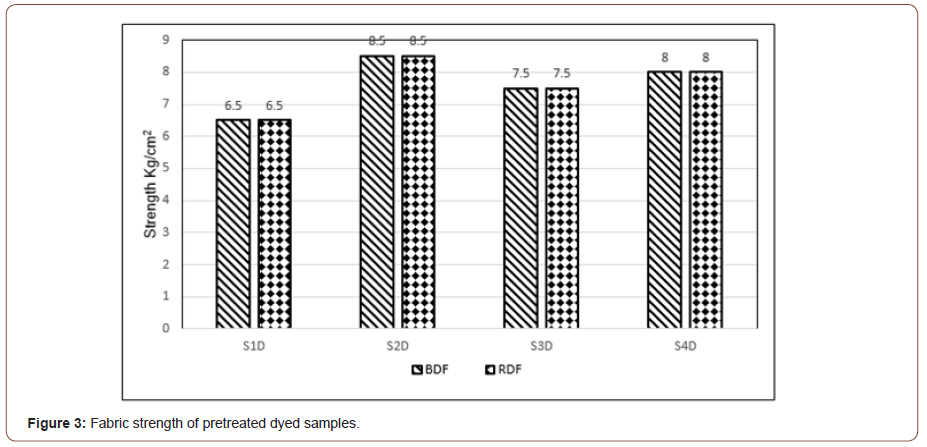
Moreover, the bar chart indicates that the bursting strength of dyed fabric is higher in the case of single stage biological pretreatment process in comparison with chemical pre-treatment process which consumes a significant amount of traditional chemicals. This indicates the advantage of the biological enzymatic pre-treatment process over the chemical treatment. It has also been observed that the bursting strength value remains identical for both dyed fabrics treated with various types of pre-treatment processes.
Fastness properties
The results of the color fastness to rubbing and washing tests on the dyed samples are shown in Tables 7 and 8. The test for the colorfastness of dyed fabric to washing was conducted according to the ISO 105-CO6:2010 (ISO, 2010) Standard in a Gyro Wash Tester (James Heal, UK). The washed sample was visually examined using the Grey Scale according to the ISO 105-A02 (for assessing colour change) and ISO 105-A03 (for assessing staining) Standards. The testing of the colour fastness of dyed samples to rubbing was performed on a Crockmeter M23888 (SDL ATLAS, Rock Hill, SC, USA) using the SIST EN ISO 105-X12:2016 standard. The greyscale was used to visually evaluate the colour fastness to rubbing.
Table 7:Representation of color fastness to rubbing.
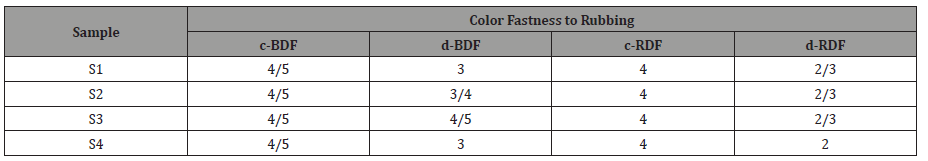
Table 8:Representation of color fastness to washing.
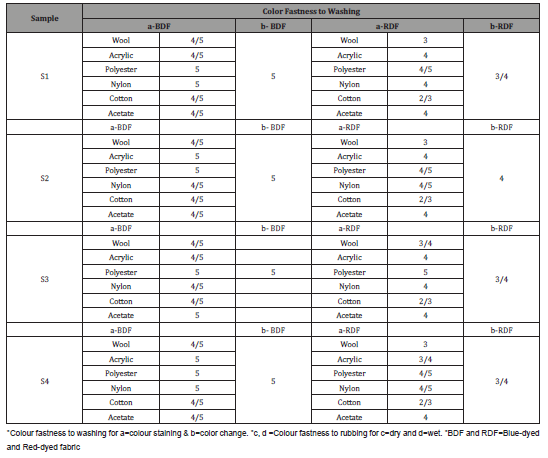
With regards of wash fastness to color staining, the highest rating in case of cotton in multifiber fabric was accounted for bluedyed fabric (4/5, very good) while the poorest rating was achieved for cold brand reactive i.e., red-dyed fabric (2/3, poor). In terms of wash fastness to colour change, the blue-dyed fabric has achieved the best result (5, excellent) but the rating of red-dyed fabric is around 3 (moderate). Overall, wash fastness (colour change and colour staining) of drimarine reactive dyed fabric is good. The rubbing fastness rating of samples S1-S4 blue dyed fabric is 4-5 (very good) in dry conditions, but it is 3/4 (average) in the wet condition. Now for cold brand dye treated, all samples show the rating of 4 (good) in dry condition but in the wet condition, the rubbing fastness of the enzymatic sample fell to 2/3 (not fast). The above result indicates that the rubbing fastness properties of the blue-dyed fabric are good.
Sustainability
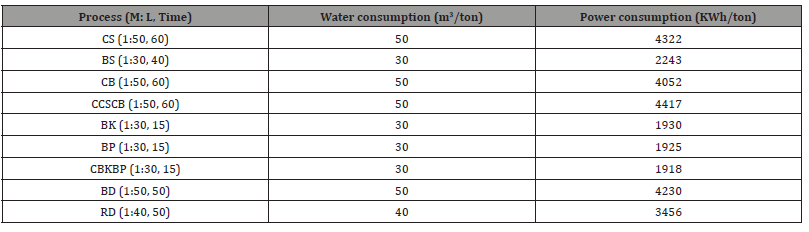
Table 9 illustrates water and power consumption per 1000 Kg of fabric for different processes. The sample S1 requires water 160 m3/ton and electricity 10,304 KWh/ton, S2 requires 140 m3/ton and 8,225 KWh/ton, S3 requires 110 m3/ton and 6,347 KWh/ton, S4 requires 110 m3/ton and 6,295 KWh/ton. The sample S4 produced from the combined biological pre-treatment process consumes the least amount of water and electricity. On the other hand, sample S1 produced from the chemical scouring and bleaching pre-treatment process consumes the highest amount of water and electricity.
Table 9:Water and power consumption per 1000 Kg of fabric.
Conclusions
Bio preparation is an enzymatic process of the cotton knitted fabric where noncellulosics impurities and surface fibers are removed by pectinases in the scouring process and by cellulase in the biopolishing process respectively. Enzymes operate in mildly favorable conditions (temperature, pH, time, water, and energy) relative to the conventional chemical-intensive process. Conventional scouring and bleaching were conducted at temperatures close to the boiling point, whereas bio scouring, bio peroxide killing, and biopolishing were performed at 60 °C.
Fabric properties in terms of bursting strength, wash fastness and rubbing fastness were better in single-stage bio-pretreated fabric than in combined bio-pretreated fabric. Combined scouring and bleaching treatments require the least amount of water and energy. Because of the reduced temperature and processing time, less energy and water were required. Therefore, the single-stage bio pretreatment process (bio scouring and biopolishing) has the potential to serve as an eco-friendly alternative to the conventional chemical pretreatment process.
Acknowledgement
None.
Conflict of Interest
No potential conflict of interest was reported by the author.
References
- Goldwaith FG, Guthrie JD (1954) Matthew's textile fibers. Wiley Interscience, NY, USA.
- Hsieh YL (2007) Chemical structure and properties of cotton. Cotton: Science and technology, pp.3-34.
- El Shafie A, Fouda MM, Hashem M (2009) One-step process for bio-scouring and peracetic acid bleaching of cotton fabric. Carbohydrate polymers 78(2): 302-308.
- Etters JN (1999) Cotton preparation with alkaline pectinase: an environmental advance. Textile Chemist and Colorist and American Dyestuff Reporter 1(3): 33-36.
- Harane RS, Adivarekar RV (2017) Sustainable processes for pre-treatment of cotton fabric. Textiles and clothing sustainability, 2(1): 1-9.
- Dasgupta J, Sikder J, Chakraborty S, Curcio S, Drioli E (2015) Remediation of textile effluents by membrane based treatment techniques: a state of the art review. Journal of environmental management 147: 55-72.
- Tzanov T, Calafell M, Guebitz GM, Cavaco-Paulo A (2001) Bio-preparation of cotton fabrics. Enzyme and Microbial Technology 29(6-7): 357-362.
- Shore J (1995) Cellulosic Dyeing, Society of Dyers and Colorists. Bradford, England.
- Buschle-Diller G, El Mogahzy Y, Inglesby MK, Zeronian SH (1998) Effects of scouring with enzymes, organic solvents, and caustic soda on the properties of hydrogen peroxide bleached cotton yarn. Textile Research Journal 68(12): 920-929.
- Mojsov K (2018) Enzymatic scouring and bleaching of cotton terry fabrics–opportunity of the improvement on some physicochemical and mechanical properties of the fabrics. Journal of natural fibers 15(5): 740-751.
- Saravanan D, Vasanthi NS, Ramachandran T (2009) A review on influential behaviour of biopolishing on dyeability and certain physico-mechanical properties of cotton fabrics. Carbohydrate polymers 76(1): 1-7.
- Bhogle S (2007) Case study on waste water disposal practices and likely treatment options in textile processing units in Tamil Nadu. TIDE, Bangalore, India.
- Varadarajan G, Venkatachalam P (2016) Sustainable textile dyeing processes. Environmental chemistry letters 14(1): 113-122.
- Cavaco-Paulo A, Gübitz G (2003) Catalysis and processing. Textile processing with enzymes, pp.89-99.
- Rahman M, Nur MG (2014) Feasible application of modern eco-friendly treatment of wool fabric before coloration. Int J Sci Res Publ 4(7): 1-7.
- Li Y, Hardin ZR (1997) Enzymatic scouring of cotton: effects on structure and properties. Cellulose 94(88): 96.
- Robner U (1993) Enzymatic Degradation of Inpurities in Cotton. Melliand, Textiberichte 74(2): 63.
- Buchert J, Pere J, Puolakka A, Nousiainen P (1998) Enzymatic scouring of cotton. In Book of Papers: 1998 AATCC International Conference and Exhibition. Philadelphia, PA, USA, pp. 493-499.
- McNeil M, Darvill AG, Fry SC, Albersheim P (1984) Structure and function of the primary cell walls of plants. Annual review of biochemistry 53(1): 625-663.
- Shen J, Smith E (2015) Enzymatic treatments for sustainable textile processing. Sustainable Apparel: 119-133.
- Nielsen PH, Kuilderd H, Zhou W, Lu X (2009) Enzyme biotechnology for sustainable textiles. In Sustainable Textiles, Woodhead Publishing, pp. 113-138.
- Tian L, Branford-White C, Wang W, Nie H, Zhu L (2012) Laccase-mediated system pretreatment to enhance the effect of hydrogen peroxide bleaching of cotton fabric. Int J Biol Macromol 50(3): 782-787.
- Arumugam K, Verenich S, Shim E, Pourdeyhimi B (2007) Pretreatment of bleached cotton fibers with whole and monocomponent cellulases for nonwoven applications. Textile Research Journal 77(10): 734-742.
-
Kazi Sirajul Islam, Mohammed Farhad Mahmud Chowdhury, Md. Sadman Sakib, Nusrat Binta Hossain, Md. Israt Bin Hossain, Mustafijur Rahman. Feasibility Analysis of the Implementation of Eco-friendly Biological Pretreatment on Cotton Knit Fabric. J Textile Sci & Fashion Tech 10(1): 2022. JTSFT.MS.ID.000726.
-
Scouring, Bleaching, Biopolishing, Dyeing, Fastness, Sustainability
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






