 Mini Review
Mini Review
A Review: Sustainable Fashion Industry Combining Supercritical Dyeing Technology with Non-Toxic Dyes
Chih-Chia Liao1 and Shen-Kung Liao2*
1The School of Medicine, Kaohsiung Medical University, Taiwan
2Department of Fiber and Composite Materials, Feng Chia University, Taiwan
Shen-Kung Liao, Department of Fiber and Composite Materials, Feng Chia University, Taiwan.
Received Date: July 12, 2021; Published Date: July 19, 2021
Abstract
The international community pays more and more attention to the responsibility of enterprises to the environment and emphasizes that their manufacturing processes. Both of product and service should continue to use overall preventive environmental strategies in order to increase ecological benefits and reduce harm to humans and the environment. We are surprised that apart from the petrochemical industry, the fashion industry turned out to be the most polluting industry in the world. There are two serious sources of pollution in the manufacturing process of glamorous fashion apparel, namely synthetic dyes and fiber dyeing wastewater. How can such pollution be improved? Microbial dyes can be used to replace synthetic dyes, and carbon dioxide gas can be used to replace precious water resources. The sustainable development of the fashion industry can satisfy consumers’ demand for colorful fashion clothing by introducing sustainable environmental protection and green technology.
Keywords:Fashion; Environment; Supercritical fluid; Microbial dyes; Sustainable
Pollutants Behind the Colorful Fashion
Clothes that are indispensable in daily life, seemingly ordinary, are actually not simple. To make a piece of clothing, from dyeing to final processing, as many as a thousand chemicals may be used. The complicated manufacturing process is as follows:
Life Process from fiber to fashion apparel
• Fiber→spinning→weaving→fabrics inspection→fabrics seam→presetting→singeing→desizing→scouring→mererization→ bleaching or fluorescent whitening→dyeing→fabric finishing→ textile design→clothing→ commodity marketing→consumers→ waste clothes landfill incineration or biodegradation
Fashion apparel retail brands will increase the number of new styles each season. Some fashion brands even launch innovative apparel several times a week. This is a kind of “fast fashion” where things are cheap, and you just throw them away after you wear them. There are 100 billion new garments produced with new fabrics every year, and many of them are thrown into landfills soon. Human intellect constantly reminds oneself that the fashion economy should be developed under the premise of protecting the global environment, but the fashion industry is listed as the world’s second most polluting industry except the petrochemical industry. How much water does it take to make a cotton T-shirt from planting cotton, weaving, dyeing and finishing to production? The answer is 2720 liters. Assuming that every person drink 2.5 liters of water a day, enough to drink for three years, scientists surprisingly discovered that clothes are also one of the prime culprits of destroying marine ecology. How much social cost is hidden behind the gorgeous clothing under the window? When we look at “fashion” more deeply and exploring the charm of the fashion industry, how much unnecessary waste, pollution and destruction does the earth bear because of the name of “fashion”?
On average, the global production of textiles for the dyeing and finishing industry is about 28 billion kilograms per year. For the dyeing and finishing part alone, it is estimated that 100 to 150 liters of water are required to produce 1 kilogram of textiles. Therefore, the textile industry is considered to be an industry that consumes a lot of water resources and highly polluting wastewater. As a member of consumers, what can we do? Understand the production process of the product, buy high-quality and durable products, and choose green environmental protection. Manufacturers should also introduce sustainable, friendly and green technologies into the fashion industry to satisfy the needs of consumers for colorful and fashion apparel.
Supercritical Fluid Dyeing Technology
Wastewater from the textile industries will seriously pollute the soil, water and the environment. As the global will is rising on sustainable environmental protection, the textile industry is concerned about the waste caused by the impact on the environment in the 21st century. Threats and destruction are also social costs and responsibilities that the textile industry needs to face for the sustainable development. International brands demand green and environmentally products actively. The dyeing and finishing section with high water consumption and high pollution in the textile process has to be transformed and upgraded. It is a major breakthrough by supercritical fluid technology which applied to textile dyeing and finishing. This green technology uses carbon dioxide as the dyeing medium, not only does not waste water at all, but the carbon dioxide used as the medium can also be completely recycled.
Supercritical fluid dyeing processes is divided into four steps including dissolve the dye: the supercritical carbon dioxide fluid will dissolve the dye molecules and diffuse into the reaction tank, surface adsorption: the dye will quickly be adsorbed on the surface of the fiber, inner layer diffusion: the dye will quickly penetrate from the surface of the fiber to the inside of the fiber, and dye-fixation: when the dye concentration on the fiber and the dye concentration in the fluid reach equilibrium, finally the fixation is formed to complete the dyeing, the process as shown in Figure 1. Supercritical fluids are suitable as solvents in industrial and laboratory processes and can replace many organic solvents. When approaching the critical point, a small change in pressure or temperature will cause a large change in fluid density. Now carbon dioxide and water are the most commonly used supercritical fluids. In the process of carbon dioxide dyeing, you can dye and remove excess dye in equipment. In the final stage, only need to reduce the pressure to release carbon dioxide and dry dyed fabrics can be obtained without drying process.
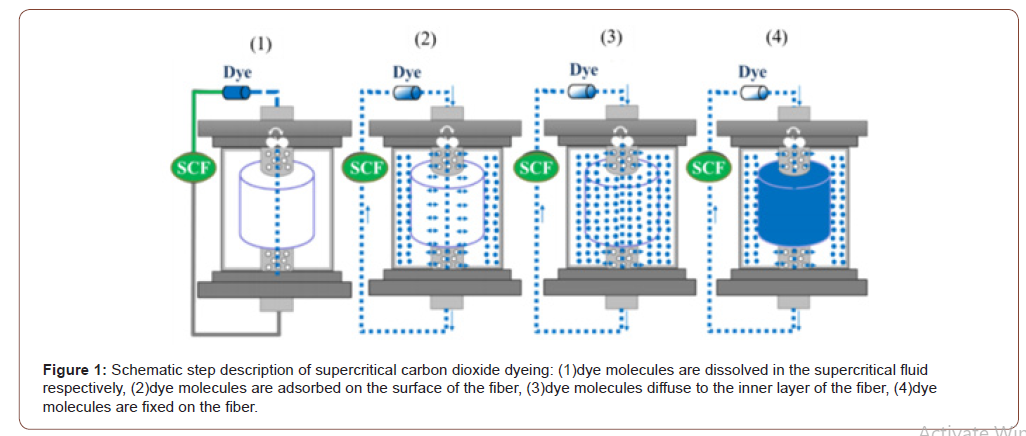
In other words, the supercritical fluid dyeing technology uses carbon dioxide instead of water. When carbon dioxide exceeds its critical temperature and critical pressure, the gas-liquid interface disappears and forms a fluid state, which becomes the so-called supercritical fluid. The operating principle is to use dyes that can be dissolved in supercritical carbon dioxide in order to dye fibers. When carbon dioxide is in a supercritical fluid state with a temperature higher than 31.1 °C (absolute temperature 304.1 °K) and a pressure higher than 7.28 atmospheres (7.38bar), the dye molecules gradually diffuse into the fiber through supercritical carbon dioxide [1]. After dyeing is completed, when the dyeing tank is returned to normal pressure (1.0 atmosphere), carbon dioxide and dye will automatically form a solid-gas separation state, dye molecules (solid) and carbon dioxide (gaseous). At this time, both of dye and carbon dioxide can be completely recycled and can be reused, so that the dyeing and finishing process can reach the highest level of pollution-free and waste-free.
This technology not only enables dyeing to achieve zero water usage, but also effectively reduces the cost of 68% textile chemicals and 34% energy. The carbon dioxide can be completely recycled and 100% reused, which is in line with today’s circular economy and friendly production trends. Contribute to the achievement of a zero-emission Roadmap plan. Industrial Technology Research Institute in Taiwan has taken the lead in developing and integrating supercritical fluid dyeing/functionalization synchronization technology. This technology was recognized by the R&D100 Awards in 2018. Dyeing and finishing of factories eliminate the use of water and fundamentally solves the environmental protection technology under the prevention and control of dyeing wastewater pollution. Not only is this technology regarded as an important milestone in the textile industry’s innovative technology in the textile industry, Textile industry in Taiwan accounts for more than 75% of the world’s supercritical fluid dyeing capacity. It is also an important development center for supercritical fluid dyeing technology in the world [2].
In fact, as early as 1879, two scholars, J.B. Hannay and James Hogarth, have discovered that supercritical fluids have excellent dissolving power. They had predicted that supercritical fluids will be an excellent solvent that can be used in industry. And after the second energy crisis, the supercritical fluid technology has risen again and has attracted the attention of the industry. Its principle is to raise or pressurize a specific fluid substance to keep the fluid at its critical pressure and critical temperature. Above, fluid substances can be made into supercritical fluids. Supercritical fluids have higher density than gases, as shown in Table 1 [3], so the solubility of solutes is higher than that of ordinary gases. That is to say, the properties of supercritical fluids between gas and liquid, using carbon dioxide supercritical fluid, have the largest critical solubility, as shown in Table 2 [4]. However, the earliest application of supercritical fluid solubility to fiber dyeing was a study jointly published by Wolfgang Saus, Dierk Knittel, and Eckhard Schollmeyer in Germany. The experimental results showed that the solubility of disperse dyes by carbon dioxide and supercritical fluids are easier to permeate than water. To the characteristics of the fiber, the polyester fiber can be successfully dyed without adding any additives, and good leveling and dyeing fastness can be directly obtained [5,6].
Table 1:Density, diffusivity and viscosity for typical liquids, gases and supercritical fluids (Comparison of gases, supercritical fluids and liquids).
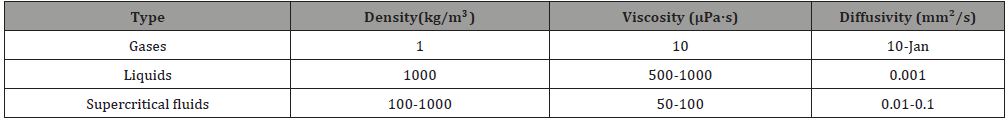
Table 2:Critical properties for some components commonly used as supercritical fluids critical properties of various solvents.
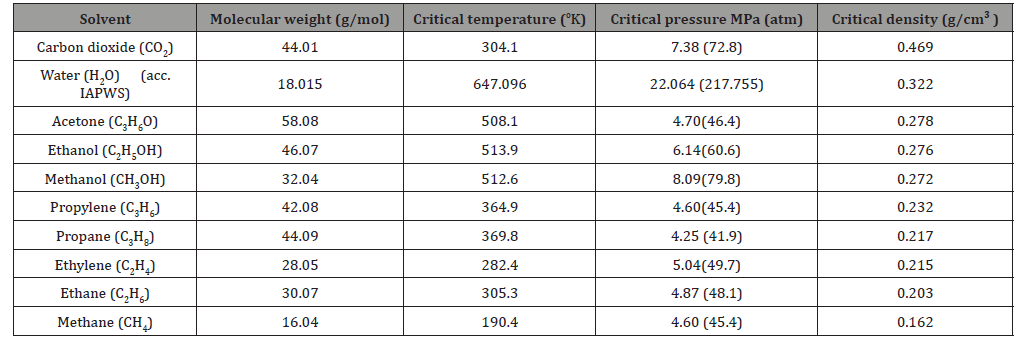
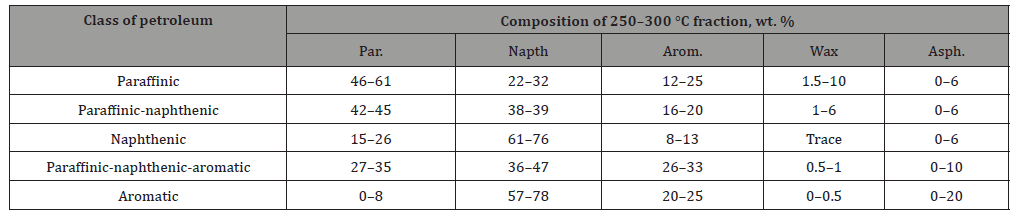
Table 3:Petroleum classification according to chemical composition.
Natural Plant Dyes and Bio-Dyes Preparation
Nowadays, the fast fashion trend is sweeping the world. The global textile dye market reached 8 billion US dollars in 2019, and the demand exceeded 1.5 million tons with an annual growth rate of 5.9%. It is estimated that it will grow to 8.75 billion US dollars in 2023. The characteristics of fast fashion in mass production and rapid replacement have also increased the burden on the environment invisibly. Because of environmental sustainability, European and American countries have more stringent in the use of chemical raw materials for textile dyes. Compared petrochemical raw materials of chemical dyes, microorganisms and the extraction of plants have non-toxic, environmentally compatible dyes become an emerging choice with great development potential in the industry.
Research-And-Markets, a market research agency, estimated that the bio-dye market will have a compound annual growth rate of 11% from 2018 to 2024, and the market will look at 5 billion U.S. dollars in 2024. The synthetic dye industry emerged in the 19th century to meet the needs of the mass market. However, the source of synthetic dyes is the chemical raw materials obtained from underground petroleum fractionation. Table 3 shows the classification of petrochemical components [7], which not only consumes the earth’s resources, but also pollute the environment. Natural plant dyes are being valued again. Plant dyeing technology is a handicraft technique that uses natural plants to dye textiles through processes such as extraction, liquid preparation, and coloring. Plant dyeing is an ancient technique, but it was gradually replaced by chemical dyeing in the eighteenth century. With the rise of global environmental awareness, people began to pay attention to natural environmental protection. Plant dyeing is the use of plant roots, stems, flowers, leaves, fruits, etc. as materials, and the juice is used for dyeing. Therefore, the unique natural warm style of plant dyeing has once again opened people’s attention. With the continuous development of dyeing techniques, this kind of dyeing is taken from nature and can be decomposed and returned to nature after use. The use of vegetable dyes can reduce the chemical load released by wastewater [8] (Table 3).
Plant dyeing is divided into cold dyeing and heat dyeing. Most plant dyes need to be heated to a certain temperature through the dye material to be dyed. The biggest difference between “blue dyeing” in plant dyeing and other plant dyeing is that it can be dyed at room temperature. Each dyeing must go through repeated steps of oxidation, washing, and treating fabrics with a counterstain. Finally, a beautiful blue dyed fabric can be obtained. Although traditional natural dyes derived from plants are non-toxic, plants that produce pigments require a lot of land and water. The large-scale harvesting efficiency is low, which is not conducive to the earth’s ecosystem, and has the disadvantages of time-consuming, labor-intensive and incapable of mass production.
Nowadays, alternative microbial dye technology can use genetically modified strains, which can make the strains synthesize specific complex dye molecules by themselves. At the same time, most bacteria or yeast strains have the characteristics of suspension culture, which are suitable for scale up to the liquid fermentation process of ton-level and can be extracted quickly [9]. With mass-produced and stable quality textile dyes, this method can effectively solve the shortcomings of natural plant dyeing that is time-consuming and labor-intensive and cannot be mass-produced. It helps to improve the quality and source stability of dyes. Yellow Escherichia coli can give strains the ability to produce various colors using gene transfer and metabolic pathway design. And then select high-efficiency strains from hundreds of millions of strains to produce dye molecules. It only takes 2 to 3 days. It can be fermented to produce microbial dyes with a variety of colors. During the entire dye production and fiber dyeing process, there will be no harmful chemicals and wastewater pollution. It is friendly (natural plant dyes) and can be mass-produced with stable quality (synthetic dyes). In the future, all dyed fabrics will be able to use bio-engineered microbial dyeing technology to meet the needs of the colorful fashion industry without having to pay the price of environmental pollution [10].
Conclusion
Faced with the earth’s limited resources, green chemical production, and environmental protection, each of us can become an active player in the consumption cycle and promote the circular economy. From the product traceability to every purchase and every choice, there is an opportunity to change the producer and the manufacturing of the product, and promote the fairness and sustainability of the producers, consumers, and the natural environment. This is to reduce its impact on the environment. There is no phase boundary between liquid and gas in supercritical fluid state and there is also no surface tension. By changing the pressure and temperature of the fluid, the characteristics of the supercritical fluid can be fine-tuned to make it more similar to liquid or gas. Supercritical Fluid technology has potential for development in industry. Due to its high mass transfer efficiency and easy penetration into the solid micropores, it has been widely used in extraction, drying, cleaning, and impregnation, fiber dyeing and finishing processes.
Especially in the fashion clothing, two serious pollution sources are chemically synthesized dyes and fiber dyeing wastewater. Synthetic dyes can be replaced by mass-produced and stable quality microbial dyes. Fiber dyeing is replaced by supercritical fluid technology, which pressurizes carbon dioxide gas and gradually presents a nearly “liquid” state. Valuable water resources can be replaced by carbon dioxide gas and become the carrier of dyes. So, there are several advantages to replacing water with carbon dioxide. The first is not to use water, the second is to reduce the use of chemical additives, and the third is to improve the work of dyeing and finishing factories surroundings. The sustainable development of the fashion industry can satisfy consumers’ demand for colorful fashion clothing by introducing sustainable environmental protection and green technology.
Acknowledgement
None.
Conflict of Interest
Authors declare no conflict of interest.
References
- Liao SK, Ho YC, Chang PS (2000) Dyeing of nylon 66 with a disperse-reactive dye using supercritical carbon dioxide as the transport medium. J Soci Dyers and Colourists 116(12): 403-407.
- Hannay JB, Hogarth J (1879) VI. On the solubility of solids in gases. The Royal Society 29: 196-199.
- Sapkale GN, Patil SM, Surwase US, Bhatbhage PK (2010) A Review Supercritical Fluid Extraction. Int J Chem Sci 8(2): 729-743.
- Reid RC, Prausnitz JM, Poling BE (1987) The properties of gases and liquids. (5th edn), New York McGraw-Hill, USA.
- Saus W, Knittel D, Schollmeyer E (1993) Dyeing of Textiles in Supercritical Carbon Dioxide. Textile Research Journal 63(3): 135-142.
- Knittel, W.Saus, E.Schollmeyer (1993) Application of Supercritical Carbon Dioxide in Finishing Process. Journal of Textile Institute 84(4): 534-552.
- Simanzhenkov V (2014) Crude oil chemistry. CRC Press, USA, p.33.
- Bechtold T, Turcanu A, Ganglberger E, Geissler S (2003) Natural dyes in modern textile dyehouses- how to combine experiences of two centuries to meet the demands of the future? Journal of Cleaner Production 11(5): 499-509.
- Russmayer H, Marx H, Sauer M (2019) Microbial 2-butanol production with Lactobacillus diolivorans. Biotechnology for Biofuels (12): 262-272.
- Chimileski S (2017) Bacterial Dyes in Fashion. American Society of Microbiology.
-
Chih-Chia Liao, Shen-Kung Liao. A Review: Sustainable Fashion Industry Combining Supercritical Dyeing Technology with Non-Toxic Dyes. 8(5): 2021. JTSFT.MS.ID.000697.
-
Fashion, Environment, Supercritical fluid, Microbial dyes, Sustainable
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






