 Review Article
Review Article
The Mechanism of Action of Cyclophosphamide and Ifosfamide
Georg Voelcker*
Institute of Biochemistry II, Goethe University Frankfurt Medical School, Germany
Georg Voelcker, Institute of Biochemistry II, Goethe University Frankfurt Medical School, Germany.
Received Date: May 24, 2023; Published Date: June 06, 2023
Abstract
Although cyclophosphamide (CP) is one of the oldest cytostatic agents’ indispensables in the clinic, its mechanism of action was unknown until a few years ago. This was because the formation of the DNA alkylating metabolite phosphoramide mustard (PAM) in vitro was negligently extrapolated to in vivo conditions. In viitro, PAM is formed by β-elimination of acrolein from aldophosphamide (ALD), but in vivo it is formed by enzymatic cleavage of ALD to form PAM and proapoptotic 3-hydroxypropanal (HPA). A mechanism of action based on this fact is presented and confirmed by therapeutic experiments with P388 tumor-bearing CD2F1 mice.
Introduction
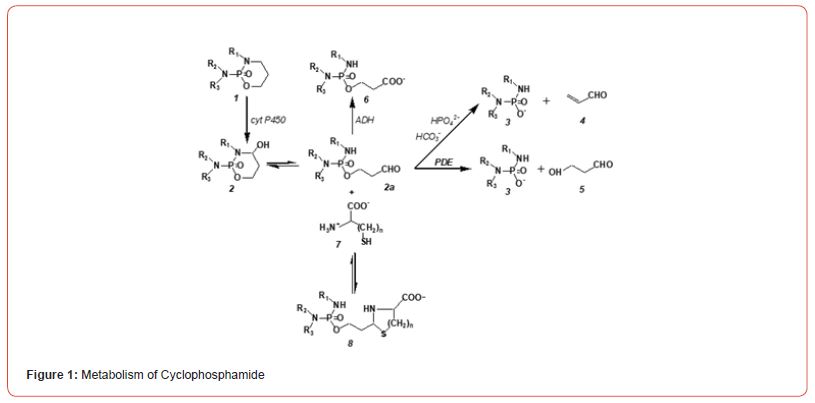
know no scientific discipline in which the increase in knowledge in recent decades has been as rapid as in biochemistry. As a result, biochemical phenomena that were inexplicable years ago can now be explained. A prime example is the mechanism of action of cyclophosphamide (CP) and other oxazaphosphorine cytostatics (OX) like Ifosfamide (IF). CP was introduced into the clinic in 1959 for the treatment of cancer, it is on the WHO’s list of essential medicines and is still indispensable in the clinic today. But nothing was known about its mechanism of action until 7 years ago. Although, according to the biochemical knowledge of the time, the metabolism of CP (figure. 1) was known. It was known that CP is hydroxylated by cytochrome P450 enzymes of the liver to 4-hydroxycyclophosphamide (CPOH), which forms an equilibrium mixture with its tautomeric aldehyde aldophosphamide (ALD), from which the actual DNA alkylating metabolite phosphoramide mustard (PAM) is released. In the formation of PAM for decades, one carelessly transferred the formation of PAM in vitro, e.g., in cell culture experiments to in vivo conditions. It was simply overlooked that in vitro PAM is formed from ALD by beta elimination of acrolein (4 figure.1), whereas in vivo it is formed by enzymatic cleavage of ALD to form 3-hydroxyprpopanal (HPA, 5 figure. 1)). This did not initially seem significant to me, when I discovered HPA as a CP metabolite [1], because DNA alkylating PAM is formed in both cases. This attitude changed abruptly with the discovery of apoptosis and the discovery of HPA as a proapoptotic aldehyde [2]. because it suddenly became clear why the therapeutic quotient of CPOH is much larger than that of PAM. (figure 1)
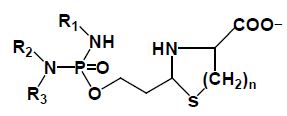
CP (1) is hydroxylated by P450 enzymes in the liver to CPOH (2), which is in equilibrium with ALD (2a), ALD is the pharmacologic active metabolite. A part of ALD is oxidized by aldehyde dehydrogenase to the non-cytotoxic carboxyphosphamid (6). The amount of ALD not oxidized is decomposed in vivo enzymatically by phosphodiesterases (PDE) to the alkylating PAM (3) and HPA (5). In vitro PAM is formed by β-elimination of acrolein from ALD. This reaction is catalyzed by bicarbonate and phosphate ions. ALD forms with β- or γ-amino thiols such as cysteine (7, n=1) or homocysteine (7, n=2) thizolidines (8, n=1) or perhydrothiazines (8, n=2).
The Mechanism of Action of Cyclophosphamide and Ifosfamide
The therapeutic quotient is a measure of the therapeutic effect of a drug. It is the ratio from the amount of a therapeutic agent that causes toxicity to the amount that causes the therapeutic effect. According to Brock [3] and Brock and Hohorst [4], the therapeutic quotient measured in Yoshida ascites sarcoma-bearing rats, was determined to be 120 for CP and CPOH but only 3.5 for PAM. This result indicates that DNA alkylations by PAM released from ALD in vivo are much more efficient than DNA alkylations produced by bare PAM injected. Although this finding had been known since 1976, efforts to improve the antitumor efficacy of CP focused on getting more alkylating PAM into tumor cells, as in the development of Glufosfamide (β-D-glucose-isophosphoreamide mustard), which has been synthesized on the basis of the rationale that cancer cells have an increased uptake of glucose. Glufosfamide was developed with the intention of enriching the alkylating moiety of Ifosfamide - that is isophosphoreamide mustard (IPAM) - in tumor cells [5]. The hoped-for increase in effectiveness did not materialize due to ignorance of the true mechanism of action.
Experiments by Iyer et al. [2], who investigated the effects of HPA on tumor necrosis factor activated apoptose signaling pathways in human leukemia cells, showed that HPA is a proapoptotic aldehyde. Among other things it inhibits the formation of the anti- apoptotic proteins Bcl-2 and Bcl-xL and the TNF-dependent NF- κB activation by inhibition of the translocation of the p65 subunit of NF-κB into the nucleus. The experiments of Iyer et al. further showed that the degradation of IκB is suppressed by HPA. Thus, by HPA apoptosis is enhanced [6]. The discovery of HPA as a CP metabolite [1], and the results published by Iyer and coworkers [2], showing that HPA is a proapoptotic aldehyde and last, but not least the scientific work of Schwartz and Waxman [7], who have demonstrated that the function of PAM is to induce cytotoxic apoptosis by DNA alkylation lead to the scheme for the mechanism of action of CP and other OX shown in (Figure 2).
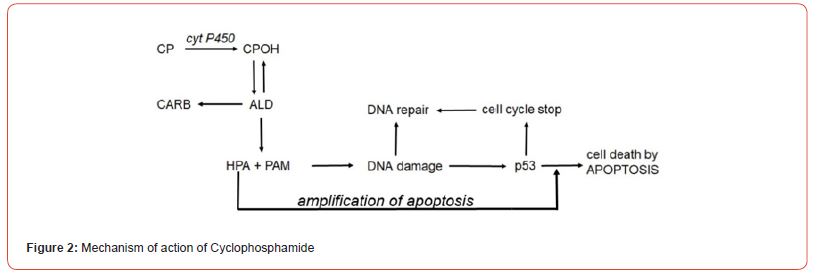
ALD is enzymatically cleaved in PAM and HPA. PAM damages DNA by alkylation. The alkylated DNA is either repaired immediately by cell owns repair processes or - if this is not possible - the tumor suppressor protein p53 is activated, which induces cell cycle stop to give the cell time to repair the damage. If DNA repair is not possible, p53 induces apoptosis, which is - and this is special for CP and IF - enhanced by HPA.
Experimental proof of the correctness of the mechanism of action presented.
According to the mechanism of action scheme (figure.2), the event leading to cell death is apoptosis initiated by DNA damage caused by PAM. The apoptosis yield is influenced by the extent of repair of the damaged DNA. Interstrand crosslinks, unlike intrastrand crosslinks, are rapidly repaired by cellular repair systems [8] and thus have a lower apoptotic yield than intrastrand crosslinks. The alkylating function of CP and IF consists of two 2-chloroethyl groups (-CH2CH2Cl) that create interstrand crosslinks and therefore have a low apoptosis yield. In contrast to 2-chloroethyl groups, 2-mesyl-ethyl groups (-CH2CH2OSO2CH3) generate intra strand crosslinks (http://www.atdbio.com/content/16/ Nucleic-acid- drug-interactions) and should therefore show a higher apoptosis yield, in plain language, better antitumor efficacy. Based on this consideration, therapy experiments were carried out in P388 tumor-bearing mice with CP and IF derivatives with 2-chloroethyl or 2 mesyl-ethyl groups in the alkylating function. If the proposed mechanism of action is correct, derivatives with 2 mesyl-ethyl groups should show significantly better antitumor activity.
Cyclophosphamide was not found by rational drug design, but by serendipity therefore it is not optimized for the mechanism of action presented. A disadvantage is the formation of the pharmacologically active metabolite ALD from CPOH because CPOH accounts for CP toxicity [9]. In order to eliminate this toxicity which masks the therapeutic effect further experiments were performed with I-aldophosphamide thiazolidines (ITIA)) and I-aldophosphamide perhydrothiazines (IAP)) (compounds 8 figure. 1), which spontaneously hydrolyze to I-aIdophosphamide (IALD) bypassing toxic 4-hydroxy-ifosfamide (IFOH). The thiazolidin and perhydothiazin derivatives are seven-to-9-fold lower toxic in mice than CPOH and IFOH [9]. The Ifosfamide derivatives were chosen because they are easy to synthesize and easy to handle. Results of therapy tests with ITIA and IAP in P388 tumor bearing CD2F1 mice are summarized in (Table 1).
Table 1:
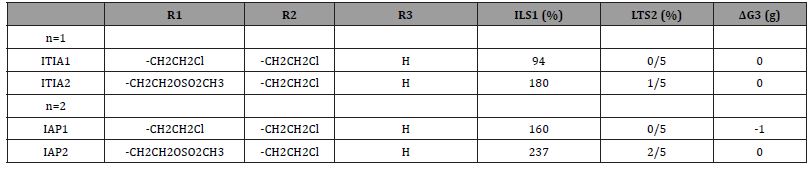
Antitumor activity and toxicity of I-aldophosphamide thiazolidines n=1 (ITIA) and Ialdophosphamide-perhydrothiazines n=2 (IAP). Female CD2F1 mice, intraperitoneal transplantation of 106 P388 mouse leukemia cells on d 0. ITIA: subcutaneous administration of 0.26 mmol/kg (equivalent to 100mg/kg ITIA1) days 1 – 5. IAP: subcutaneous administration of 0.51 mmol/kg (equivalent to 200mg/kg IAP1) days 1 – 5
1: ILS increase in life span, 2: LTS long time survivors (surviving time >100d), 3: ΔG difference in body weight at d 0 and the lowest body weight after drug administration.
The table shows that both the thizolidine and the perhydrothiazine of I-aldophosphamide, in which a 2-chloroethyl group is replaced by a mesyl-ethyl group that generates irreparable or poorly repairable DNA intrastrand crosslinks, are significantly more effective against P388 tumor bearing CD2F1 mice. With ITIA2 and IAP2 long-term survivors (surviving time >100d) and longer survival times than with ITIA1 and IAP1 are achieved. Based on body weight, the doses administered are non-toxic.
In another experiment CD2F1mice bearing advanced P388 tumors were treated with 320 mg/kg IAP1 and 266mg/kg IAP2 on day 5 after subcutaneous transplantation of 106 P388 mouse leukemia cells. The tumors had a size of about 0.5 cm2 at this time. In the mice treated with IAP, a marginal increase in life span of 60% was measured; on the other hand, the animals treated with IAP2 showed an increase in lifespan of 285%. This result only partially reflects what is seen in the experiment. To illustrate the superior antitumor effectiveness of IAP2 on the transplanted P388 tumor in CD2F1 mice, it should be mentioned that IAP2 reduced the tumor size below the detection limit from day 14 to day 30. The animals died between day 35 and day 63 from the growth of metastases in the region of the lymph nodes of the forelegs and hind legs.
Conclusion
1) The results of the work of Schwartz and Waxmann [7] who have demonstrated that the function of PAM is to induce cytotoxic p53 controlled apoptosis by DNA alkylation; the results of the work of Iyer et al [2] who showed that HPA is a proapoptotic aldehyde; and the discovery of HPA as a CP metabolite [1] have led to the postulation of the mechanism of action of CP and IF.
2) The therapy experiments with CP and IF derivatives with different alkylating functions, which produce DNA damages that are difficult or easy to repair, have confirmed the postulated mechanism of action.
Acknowledgement
None.
Conflict of Interest
None.
References
1 Voelcker, G (2017) Enzyme Catalyzed Decomposition of 4-Hydroxycyclophosphamide. Open Conf. Proceeding J 8: 44–51.
4 Brock N, Hohorst HJ (1977) The problem of specificity and selectivity of alkylating cytostatics: Studies on N-2-chlorethylamido-oxazaphosphorines. Z. Krebsforsch 88: 185–215.
-
Georg Voelcker*. The Mechanism of Action of Cyclophosphamide and Ifosfamide. Insi in Chem & Biochem. 2(4): 2023. ICBC. MS.ID.000545.
-
Materials Science, fibers, concrete, fiber-reinforced concrete, polymer, synthetic fiber, Artificial fiber.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






