 Review Article
Review Article
Study of the Role of Natural Radionuclides in Chemical Processes in Green Vegetation
Khagani Farzullaoglu Mammadov*
Institute of Radiation Problems of Ministry of Science and Education of Azerbaijan, Baku city, Russia
Khagani Farzullaoglu Mammadov, Institute of Radiation Problems of Ministry of Science and Education of Azerbaijan, Baku city, Russia
Received Date:July 7,2025; Published Date:July 16, 2025
Abstract
In fertile soils the development of green vegetation is directly proportional to the concentration of microelements and natural radionuclides, in the range of concentrations of microelements formed in the soil cover of the planet. The analysis showed the presence of natural radionuclides in all samples of water, soil, vegetation, livestock products. The high development of vegetation, green cover and trees in fertile areas is explained by the stimulation of photosynthesis process by relatively high concentrations of microelements and natural radionuclides. The fertile soils are characterized with relatively high concentrations of mineral components and natural radionuclides. These results are in good agreement with the higher degree of development of green cover, vegetation and trees observed in areas with fertile soils, which is explained both by the high fertility of the soil and by the participation of microelements and natural radionuclides in the acceleration of photosynthesis processes.
Keywords: Green Vegetation; Photosintesys; Natural Radionuclides; Water and Carbon Dioxide Mixtures; Fertile Soil; Hydrocarbons
Introduction
The main part of the soil is formed by chemical compounds in the form of various minerals. Soil is the upper layer of the lithosphere exposed to living organisms and the atmosphere. The study of various forms of the presence of chemical elements in minerals, organic residues and emissions, soil colloids, deter-mining the amounts of oxides, hydroxides, carbonates, bicarbonates, nitrates, nitrites, sulfates, and phosphates in soil samples allow us to estimate the ecological state of the soil [1-3]. Radioactive elements and heavy metals along with non-metals and light metals in the tables of chemical elements are distinguished and characterized by high density, biological activity, toxicity, ability to migrate in the habitat of living organisms through food chains “atmosphere – wind – rain – soil – plants – animals – humans” and accumulate in environmental objects and organisms [1-3].
Heavy metals participate as catalysts in numerous redox reactions, isomerization, hydration, and dehydration processes occurring in environmental objects [4-6]. Depending on the magnitude of their concentration in the body, microelements can have a “threatening, deficient, physiological, toxic and lethal” effect on the body [5-14]. It has been established by systematic studies that all environmental objects (water, soil and vegetation) contain natural radionuclides, including 40K and 22Na isotopes [15]. The study of the influence of natural radionuclides on the processes occurring in environmental objects, the role of ionizing radiation from radionuclides in the course of complex multistage processes in the vegetation cover are important sources of arguments for thepredictability of possible changes in the habitat of living organisms [8-16].
Experimental Part
The experiments were carried out under static conditions, by irradiating glass ampoules filled with the studied mixtures. The filling of ampoules with carbon dioxide, as well as the purification of water from dissolved gases, were carried out using an experimental vacuum installation. The installation consists of a vacuum part, glass volumes for storing initial liquids and gases, a measuring part (pressure gauges) that allows working up to pressures of 2.105 Pa. The glass ampoules intended for filling with the studied mixtures were previously connected to the vacuum outlet of the installation and pumped out to 102103 Pa (for 30 minutes at Т500 К, and then for another 30 minutes at room temperature), after which they were disconnected from the vacuum installation and filled with the studied systems: Н2О, Н2О – СО2, 40КCl - Н2О – СО2, 40КCl - Н2О – СО2 – “organic matrix”. Repeatedly washed with distilled water various amounts (1.0, 5.0 and 10.0 g) of green leaves of the olive tree (Olea europaea L.) were chosen as the organic matrix. Gases were evacuated by a vacuum installation equipped with vacuum lamps, traps, oil and mercury manometers, and glass vessels with three-way vacuum cocks to purify water from dissolved gases. The water used is repeatedly (4-5 times) purified according to the cycle “freezing - vacuum filling - pumping out - ice thawing - slow pumping out of dissolved gases” at 293 K [17]. Qualitative analysis of the studied components was carried out using a gas chromatograph GC-2010 (Shimadzu, Japan) at a temperature of 398 K, with a flame ionization detector (FID) connected to a Supelco Nucol capillary column (30m×0.32mm). Helium (99.995%) was used as a carrier gas. The radiolysis products (H2, CO, CH4) were analyzed by chromatography at room temperature on a “GasoChrome 3101” gas analyzer equipped with a thermochemical detector and a packed column (3 m × 3 mm) filled with activated carbon at the factory. The flow of atmospheric air forced by the factory mini-pump of the device was used as a carrier gas. Hydrocarbons were analyzed also, on an Agilent 7890A chromatograph (Agilent, USA) equipped with two detectors for operation in two modes: for the analysis of hydrocarbons at a temperature of 398 K with a FID connected to a capillary column (30 m × 0.32 mm) GC-Gaspro, as well as for hydrogen analysis at room temperature with a katharometer connected to a capillary column (30 m×0.53 mm) Supelco CarboxenTM. Helium (99.995%) was used as a carrier gas in both modes.
Irradiation of glass ampoules filled with test mixtures was carried out with ionizing gamma radiation from 60Co sources of a powerful gamma installation “УК-120000”. The dose rate absorbed in the irradiation zone was 6.6 kRad/hour (0.066 kGy/hour). The soil samples taken were treated with distilled water, weak solutions of acid and alkali with periodic mixing and filtration, isolation of sparingly soluble particles in a centrifuge with further evaporation to obtain minerals, heavy metals and radionuclides. After radiometric measurements, the obtained dry mineral was analyzed by analytical chemistry, X-ray fluorescence, gamma, beta and atomic absorption spectroscopies and electron microscopy. Radiometric measurements were carried out using the İnSpector-1000 and Radiagem-2000 radiometers (manufactured by Canberra and equipped with alpha, beta and gamma detectors) and the radiometer Identifier (Thermo Scientific). In the process of physical-chemical analysis of minerals obtained by evaporation of aqueous, weakly acid and weakly alkaline extracts of soil samples by treatment of plant samples by nitric acid’s solution and heat treatment were use gamma spectrometer with HP-Ge detector manufactured by Canberra, Electronic Microscope “SEM ”(manufactured by Carl-Zeiss with an electron tube), atomic absorption AA-6800 spectrometer (manufactured by Shimadzu), Expert-3L and XRF X-ray fluorescence spectrometers [14-16].
The Results Obtained and Their Discussion
The employees of our laboratory carried out a systematic observations and study of the regions of our country, soils fertile areas, examination of green vegetation and determination of geometric dimensions and biological parameters. The common concentrations of components in the composition of soil samples taken from country’s fertile areas is 7.62-10.19 g/kg and common concentrations of components in the composition of soil samples taken from other non-fertile areas of country is 5.47-7.15 g/kg. The value of the radioactive background in country’s fertile areas is 0.03-0.15 μSv/hour and the intensity of alpha radiation is 0-0.03 Bq/cm2. The value of the radioactive background in other nonfertile soil areas of country is 0.08 μSv/hour and the intensity of alpha radiation is 0-0.01 Bq/cm2. A comparative analysis of the concentrations of mineral components and natural radionuclides shows that fertile soils are characterized by relatively high concentrations of the studied components. The energy of gamma rays (1.45 MeV) emitted by the K40 isotope is many times higher than the value of the binding energy of hydrogen with a hydroxyl group in water molecules (5 eV). In addition, K40 isotopes were found in all samples without exception taken from the environment, and the geometric dimensions of the studied vegetation specimens were directly proportional to the activity (concentration) of K40 detected in them. The high energy of gamma rays irradiated with K40 and the relatively high activity are the reason for the increase in the current concentration of radicals in the mass of the plant, which is equivalent to the acceleration of high-barrier endothermic process of the splitting of hydrogen atoms from water molecules. The analysis of numerous samples of water, soil, vegetation, livestock products showed the presence of Na22 and K40 radioisotopes in all samples, without exception. A comparative analysis carried out using physical chemistry methods shows that the fertile soils of country’s areas are characterized by relatively high concentrations of mineral components and natural radionuclides. Conducted analyzes of the mineral composition and appearance of vegetation in different areas of green plants, as well as a comparison of the appearance of green spaces grown in experimental areas (on soil with a natural activity of 40K 0.8 Bq / kg and on soil impregnated with an aqueous solution of 40KCl salt with an activity of 40K brought to 2.5 Bq/kg) showed a relatively high growth rate of vegetation in soil areas containing high concentrations of 40KCl (see Figure 1).

The course of photosynthesis is described by the overall equation:
6CO2 + 6H2O → C6H12O6 + 6O2 (1)
The high endothermicity (3080 kJ/mol) of this reaction is due to the high values of the dissociation energy of the O–H bond (485- 498 kJ/mol or 5.0–5.2 eV/molec.) in the water molecule and the C=O bond (799 kJ/mol or 8.3 eV/molec.) of carbon dioxide molecule [11,12]. Low energies of visible light quanta (below 5 eV/quantum) are not sufficient to break the C=O bond. However, the high energies of gamma radiation quanta from the 40K isotope easily explain the dissociation of carbon dioxide and water molecules into the corresponding radicals and ions. The presence of 40K isotopes in all environmental objects (in water, soil and vegetation), relatively high growth rates of plants in soil areas containing relatively high concentrations of 40KCl, the presence of photosynthesis variants on the cytoplasmic membrane of extreme halobacteria or in the absence of chlorophyll and oxygen, in the depths of large lakes and seas of the planet in the presence of long-wavelength infrared radiation (from volcanic lava) testify in favor of the expediency of taking into account the role of ionizing radiation from 40K at the initial energy-intensive stage of photosynthesis, i.e. dissociation of CO2 and H2O molecules [11].
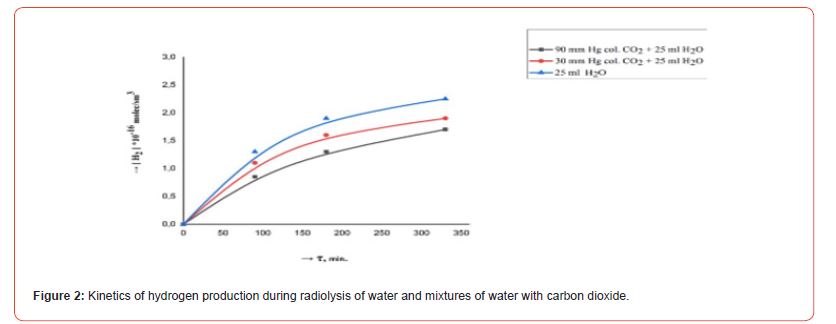
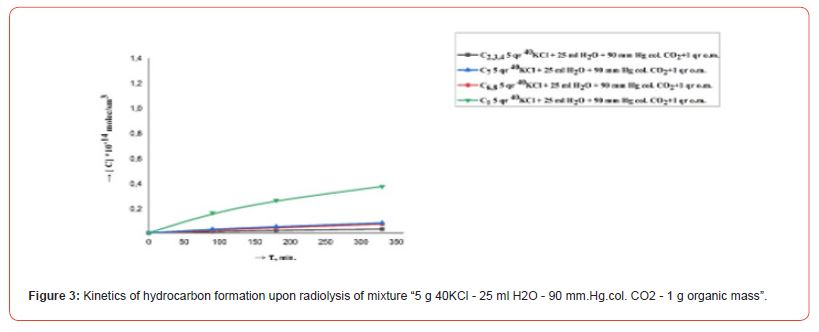
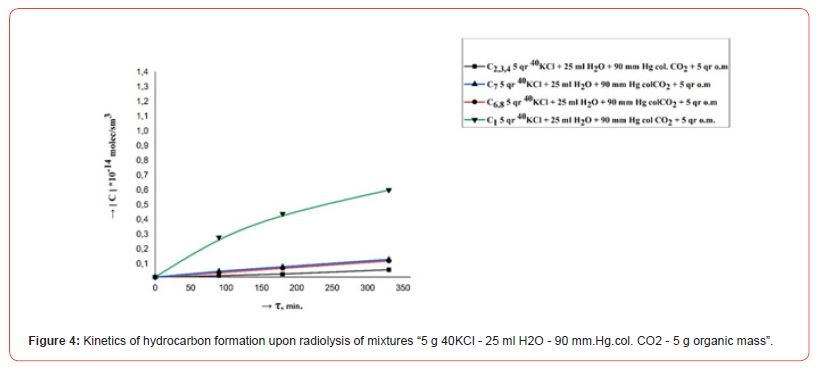
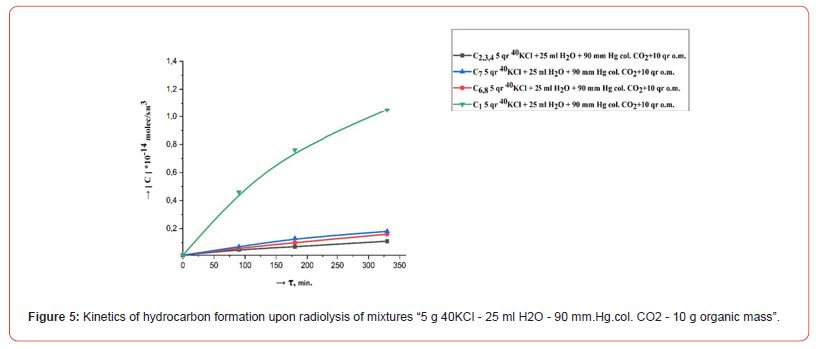
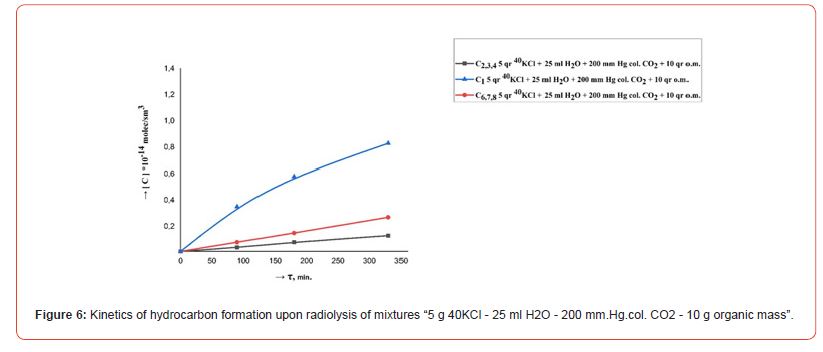
The kinetics of the formation of radiolysis products in the systems H2O, H2O - CO2, 40KCl - H2O - CO2, 40KCl - H2O - CO2 - “organic mass” under the influence of ionizing gamma radiation were studied in order to research the course of chemical processes in a mixture of water with carbon dioxide in the presence of mass green vegetation. Repeatedly washed with distilled water different amounts (1.0, 5.0 and 10.0 g) of green leaves of olive trees (Olea europaea L.) were chosen as the organic matrix. Conducted radiometric measurements showed that the activity of radiation from 5.0 gr. 40KCl salt in one ampoule was 40 Bq. Filled with the indicated mixtures ampouyles before irradiation at a powerful gamma installation were stored for 30 days around a vessel containing a weak source of gamma radiation (filled with 40KCl salt) with an activity of 1000 Bq. Figures 2, 3, 4, 5 and 6 show the kinetics of the formation of radiolysis products of the studied mixtures irradiated at the high-power gamma installation УК- 12000.
Hydrogen formation was not observed during chromatographic analysis in ampoules filled with water and a mixture of water with carbon dioxide, which were stored for a month around a weak source with an activity of 1000 Bq. However, the presence of a trace amount of hydrogen was detected in ampoules containing multi-component systems stored for 30 days near a weak radiation source. This amount ((0.1-0.2) × 1014 molecules/cm3) was many times (more than 106 times) lower than the values of the hydrogen concentration (indicated on the kinetic curves in Figure 4) formed in the ampoule under the action of ionizing radiation from the powerful gamma installation УК-120000. The radiation-chemical yield of hydrogen formation from water is 0.3–1.0 molecules/100 eV [12]. Gamma rays emitted by a source with an activity of 1000 Bq emit a total energy over 30 days of 1000 × 30 × 24 × 3600 × 1450000 eV = 3.8×1015 eV, which corresponds to the formation of no more than 0.4×1014 H2 molecules from pure water in an ampoule during 30 days. The formation (radiation-chemical yield) of hydrogen during the radiolysis of organic matter is approximately ten times higher, which corresponds to the formation of no more than 4.0 × 1014 molecules or 0.1 × 1014 H2/cm3 molecules in the ampoule, taking into account that the volume of the ampoule is 40 cm3.
The value obtained by calculation explains the detected “dark effect of the formation of trace amounts of hydrogen” in ampoules containing mixtures of “40KCl-H2O-CO2-organic mass”.
The study of the kinetics of the formation of products during the radiolysis of the above systems led to the following conclusions:
• The rate of CO formation increases with an increase in the concentration (pressure) of CO2 in the H2O – CO2 mixture, the rate of H2 formation decreases and the formation of hydrocarbons is not observed in this case.
• The rate of formation of CO with an increase in the concentration (pressure) of CO2 from 30 to 200 mm. Hg. col. in mixtures “5 g 40KCl - 25 ml H2O - CO2 – 1.0, 5.0 and 10.0 g organic matrix” increases, and the rates of formation of H2 and CH4 decrease. At the same time, the total amount of formed relatively heavy hydrocarbons (С2-С8) does not change significantly.
• An increase in the rate of formation of H2, CO, CH4 and relatively heavy hydrocarbons (C2-C8) is observed with an increase in the amount of organic matter in mixtures “5 g 40KCl - 25 ml Н2О – 90 mm. Hg. col. CO2 – organic matrix.
• The rate of formation of molecular products with an increase in the absorbed dose of radiation in the system “5 g 40KCl - 25 ml H2O - 30 mm. Hg. col. CO2 - 10 g organic matrix” do not change significantly and the accumulation of products is observed in proportion to the value of the absorbed dose.
The revealed regularities well explain the observed high growth rates of vegetation in soil areas containing high concentrations of 40KCl, which are micro sources of ionizing radiation thatcreate relatively higher doses than in similar soil with vegetation (with relatively low growth rates) containing relatively low concentrations of 40KCl. The rapid growth and maturation of fruits of figs (Ficus carica L.), white mulberries (Morus alba L.), after a certain growth phase, the accumulation of glucose and fructose in them during summer days and nights, testifies in favor of the proposed version. The formation of heavier hydrocarbons and carbohydrates in the studied multicomponent systems under the influence of ionizing rays can be explained by the mechanism of formation of heavy molecules, described in detail in [13,14] which consists of a sequence of numerous elementary reactions with a number of values of their rate constants indicated.
Conclusions
The rate of decrease of molecular hydrogen formation with an increase in the concentration of CO2 is observed during the radiolysis of mixture of vapors of H2O - CO2. The rates of formation of all products (H2, CO, CH4, and relatively heavy hydrocarbons) increase with increasing absorbed dose and amount of organic matter in mixtures with stable concentrations of CO2, H2O, and 40KCl. There is a decrease in the rate of formation of H2 and CH4, and an increase in the rate of elementary reactions of the transformation of light radiolysis products (H2, CO, CH4) into relatively heavy products (C6, C7, C8) with an increase in CO2 concentration. The considered and obtained data (the presence of 40K isotopes in all environmental objects, the efficiency of the process of assimilation of 40K from water by plants, the relatively high rates of plant development in soil areas containing high concentrations of 40KCl, the presence of photosynthesis in the absence of chlorophyll and oxygen, on the cytoplasmic membrane of extreme halobacteria, in the depths of large lakes and seas of the planet in the presence of long-wavelength infrared radiation /from volcanic lava/, qualitative changes in the composition of fruits of figs /Ficus carica L./, white mulberries /Morus alba L./ both in summer days and at night, the high value of the C=O bond energy in carbon dioxide molecules, which is much higher than the energy of visible light quanta) indicates the expediency of taking into account the role of ionizing radiation from 40K at the initial energy-intensive stage of initiating the dissociation of molecules (CO2, H2O, etc.) when considering traditional multistage biochemical mechanisms of photosynthesis.
Conflicts of Interest
The author declares no conflicts of interest regarding the publication of this paper.
References
- Filgueiras AV, Lavilla I, Bendicho C (2002) Chemical sequential extraction for metal partitioning in environmental solid samples. Journal of Environmental Monitoring 4(6): 823-857.
- Daniel GS (2021) Sorption mechanisms of chemicals in soils by department of soil and water systems. Soil Syst 5: 1-13.
- Nadporozhskaya M, Kovsh N, Paolesse R, Larisa Lvova L (2022) Recent Advances in Chemical Sensors for Soil Analysis: A Review. Journal Chemosensors 10(1): 35.
- Mollan AS, Rahman MM, Husain SR (1986) Distribution of Y-Emitting Radionuclides in Soils at the Atomic Energy Research Establishment, Savor, Bangladesh. Health Physics 50: 85-88.
- Kaushik A, Kansal A, Kumari S, Kaushik CP (2009) Heavy metal contamination of river Yamuna, Haryana, India: assessment by metal enrichment factor of the sediments. Journal of Hazardous Materials 64 (1): 265.
- Qiaoqiao Z, Nan Y, Youzhi L, Bo R, Xiaohui D, et al. (2020) Total concentration and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glabal Ecology and Conservation 22: 11.
- Akhmetkaliyeva MSh, Sassykova LR, Aubakirov YA, Sendilvelan S (2017) Heavy metals accumulation by the vegeta-tion of the territory of the East Kazakhstan. International Journal of Biology and Chemistry 10(2): 40.
- Al-Sabti K, Metcalfe C (1995) Fish micronuclei for assessing genotoxicity in water. Mutation research 343(2): 121-135.
- Mammadov, Kh (2012) Radiolytic Destruction of Aflatoxin in Damp Mixed Fodders. Proceedings of 12th World Congress on Environmental Health, Vilnius 23-25.
- Mammadov Kh (2012) Radiolytical Destruction of Nivalenole in Pumpkin Seeds. Scientific Notes of the Crimean Federal University Named after VI Vernadsky. Biology Chemistry 25: 289-293.
- Mamedov KhF (2012) Radiolytic Decomposition of Zearalenon in Wheat Grains. Journal Immunopathology, Allergy Infectology 1: 74-77.
- Mamedov KhF, Aliev AG, Mamedova NA, Badalova AR (2015) Radiolysis of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin in Water Solutions. Journal of Physical Chemstry A 89: 1707-1709.
- Mamedov KhF (2014) Radiolytic Conversion of Natural Toxins in Contaminated Plant Products and Aqueous Solutions. Scientific Journal Science Rise 4: 116-121.
- SakumotoA, Miyata T (1984) Treatment of Waste Water by a Combined Technique of Radiation and Conventional Method. Radiation Physics and Chemistry 24(1): 99-115.
- Mammadov Kh (2020) The Initiators of the Appearance of Organic Compounds on Eart, the Distribution of Natural Radionuclides in the Plant Mass. Journal of Energy, Environmental & Chemical Engineering. USA. SciencePG 6(1): 16-23.
- Mammadov Kh, Shiraliyeva H, Mehtiyev E, Aliyeva-Jabbarly U, Guliyev E, et al. (2021) Study of purification processes of soil contaminated with uranil nitrate. Journal of Problems of Atomic Sciences and Technology 133(3): 132.
- Kurbanov MA, Mamedov KhF, Rustamov VR (1988) The chain formation of hydrogen during thermo-radiolysis of gas mixtures of H2S-CO. J. Chemistry of High Energies 22(3): 218.
-
Khagani Farzullaoglu Mammadov*. Study of the Role of Natural Radionuclides in Chemical Processes in Green Vegetation. Insi in Chem & Biochem. 3(3): 2025. ICBC. MS.ID.000561.
-
Green Vegetation; Photosintesys; Natural Radionuclides; Water and Carbon Dioxide Mixtures; Fertile Soil; Hydrocarbons
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






