 Research Article
Research Article
Respiratory Support Adequacy for Very Low Birth Weight Infants Post Extubation
Masoud Rasha, Pediatric Department, NICU, Tawam Hospital, Abu Dhabi Health Services Company, UAE.
Received Date: February 19, 2020; Published Date: March 05, 2020
Abstract
Objective: Study the predictors of extubation trial failure, for very low birth weight infant.
Methods: Retrospective review and analysis of very low birth weight infants, intubated in neonatal intensive care unit - Tawam Hospital, 2011 till 2018.
Results: GA (21+4- 26+6) weeks, extubation failure associates Male gender, FIO2 level >0.30, and high PCO2 level above 55 mmhg post extubation, Ground glass appearance on chest XR at trial, large PDA, low and advanced grades IVH. Success associates FIO2 level <(0.25) before extubation, post extubation PEEP level (6-7) cm water.
GA (27- 29+6) weeks, failure associates FIO2 level >0.30, and low PH level (6.9 - 7.24) post extubation, advanced grade IVH. Success associates single antenatal steroid dose, FIO2 level (0.21) before extubation, post extubation PEEP level range (6-7) cm.
GA (30- 32) weeks, failure associates FIO2 level >0.30, low PH level (6.9 - 7.24), and low PO2 (20-40) mmhg post extubation, large PDA. Success associates mother’s Pre-labor rupture of membranes, adequately sized, waiting until FIO2 requirement level is < (0.25), and IT >0.38 sec before extubation.
Surfactant dose not determine extubation trial results for GA (21+4- 23+6). Success associates 2 doses for GA (24- 26+6) weeks, single dose for GA (27- 29+6) weeks, and all 1st, 2nd and 3rd doses for GA (30-32) weeks.
Low phosphorus level associate’s failure for GA (24- 26+6, 27- 29+6).
Conclusion: Predictors of extubation trial failure vary among GA groups, and may guide physician to predict the result of extubation trial and reduce exposure to failure. Optimizing documentation and follow up research studies on larger sample size, are recommended to analyze secondary predictors.
Keywords: ELBW; Extubation failure; Risk factor; Bronchopulmonary dysplasia; Ventilation; NAVA
Background
Antenatal steroids and early use of nasal continuous positive airway pressure (NCPAP) have significantly improved outcomes of very low birth weight infants with respiratory distress syndrome [1,2], but intubation with ventilator support is still required and the optimal timing of extubation remains unclear. Nearly two thirds of premature born at less than 29 week gestation require mechanical ventilation during NICU stay [3]. Acute complications, and bronchopulmonary dysplasia (BPD), adverse neurodevelopmental outcomes, related to mechanical ventilation and endotracheal intubation in extreme preterm infants [4,5] are encouraging physicians to extubate infants as early as possible. Short-term (less than 7 days) mechanical ventilation itself is known to be a cause of rapid diaphragmatic dysfunction, and long-term ventilation (more than 12 days) is also associated with failure of normal pulmonary growth and maturation [6]. But still 40% of mechanically ventilated ELBW infants require re-intubation following extubation [7]. Failure of extubation has been associated with higher mortality rates, increased length of hospital stays, and longer ventilation days [8,9].
Mode of ventilation that converts electrical activity of diaphragm into proportionally assisted and synchronized breath is known as neutrally adjusted ventilatory assist (NAVA) [6]. Infants inform the neonatologist of what support they need, directing both the timing and depth of their breath pattern [10]. NIV NAVA can provide synchronized post extubation ventilatory support as measured by decreased PCO2 in premature infant [11].
NAVA appears to work well in neonates, but if NAVA makes a difference in outcomes in this population, has not been established so far [10].
Methods
Research proposal was reviewed and approved by Tawam Human Research Ethics Committee-Abu Dhabi Health service company UAE. The purpose of the study is to evaluate the rate and predictors of extubation failure in VLBW, especially the post extubation ventilation set to establish a local protocol to avoid extubation failure, improve the outcome of VLBW infants, and decrease the rate of chronic lung disease. Hospital ID number was de-identified and masked to secure confidentiality and no consent was required, the study was in compliance with the Declaration of Helsinki. The study work starts by a pharmacy list of 1580 shot of surfactant 2011- 2018, given to 761 neonates admitted to NICU, as some patients require 1st, 2nd, 3rd or 4th doses of surfactant. Retrospectively we collect Data from records of 457 neonates who fit criteria of <32 weeks GA, or < 1500g BWT. 11 neonates are excluded for congenital anomaly.
We evaluate 446 VLBW admitted to neonatal intensive care unit (NICU) –Tawam Hospital and intubated from Jan 2011 till Jan 2018. Risk factors, ventilation parameters, blood gas results prior and 2 hours post extubation trial are analyzed. Definition of extubation failure is re-intubation within 5 days due to attending physician assessment of clinical status and blood gas. Over years of the study some of NICU team turned over (Guide for extubation is the aim of the study), causing bias that could not be handled retrospectively. Tool of statistical analysis is IBM SPSS Statistics version 20. We use non-parametric tests for non-normally distributed Data. Categorical variables analysis is loglinear and 3way Pearson’s Chi square X2. The test analyzes association between extubation trial result and each predictor for each GA group GA (21+4- 23+6, 24- 26+6, 27-29+6, 30-32), but have not allow for analysis of all predictors at once. At some steps analysis we must merge GA groups (21+4- 23+6, 24- 26+6) for small sample size of GA group (21+4- 23+6).
Extubation results are 1- success, 2- fail, 3- (not fit for extubation till end of 14 days of life). As not fit for extubation and failing it share the case of VLBW undue for extubation, We have merge 2 and 3 for part of predictors, while used (Success, Fail) for ventilation predictors. Goodness of fit tests and cramer’s V determines the strength of association. We run several steps analyses for some predictors to extract cut off significance or compare several variables in pairs. Prenatal Steroid is grouped (No steroid given or unknown, Steroid given) then subgrouped into (1 dose, 2 doses). Timing of each dose is not recorded. Size for GA groups (small for GA- below 10th centile on Fenton growth chart, Adequate for GA, Large for GAabove 90th centile on Fenton growth chart). Gender groups (Male, Female). Number of surfactant doses is determined by physician’s opinion and clinical requirement. Patients who died at 1st day of life are excluded from surfactant analysis. The test is performed on 4 levels to test the cut off statistically significant dose for each GA. Gestational diabetic status GDM groups (No GDM or unknown, GDM). Mother’s chorioamnionitis status before delivery is defined by gynecologist clinical assessment only and follow up histologic chorioamnionitis is not recorded. Chorioamnionitis groups (No chorioamnionitis or unknown, chorioamnionitis). Pre-labor ruptured membrane mother’s status groups (No PROM or unknown, PROM >18hours). Caffeine treatment groups (no Caffeine, Caffeine). Phosphorous level at 2nd week of life groups (less than 1.8 mmol/L, above 1.8 mmol/L). Day of life at 1st intubation (Intubated 1 in delivery room, any time later). Ventilation Mode before extubation trial groups (NAVA, Conventional ventilation +/- Volume targeted ventilation, PSV). Ventilation parameters before extubation are set by attending physician. Peak Inspiratory Pressure (PIP) level between (8-28) cm H2O, Median 18.8 cm H2O, Mode 20 cm H2O. Positive end-expiratory pressure (PEEP) level between (5-8) cm H2O. PEEP groups(<6, >6) cm H2O. Ventilation Rate 20-60/min, “mode 40/min”. Rate groups (20-40, 41-60)/ min.
Fraction of inspired oxygen FIO2 Mode is 0.21. Inspiratory time (IT) range (0.20-0.86) sec. Mode 0.38 sec. IT groups (0.20-0.33, 0.33-0.37, 0.38-0.4, 0.4-0.86) sec. Patient’s respiratory rate before extubation (RR) range (20-99)/min, Mode 50/min. RR groups (20- 40, 40-70, 70-99)/min. . Blood gases are capillary and arterial samples and we set groups following acid-base homeostasis range of arterial blood gas of VLBW. PH mean 7.33 (7.0-7.57), PH groups (7- 7.24, 7.25-7.34, 7.35-7.57).
PCO2 mean 41 (9.2-69) mmhg, PCO2 groups into (9-34, 35-54, 55-70) mmhg. PO2 mean 49.4 (21.5-144), PO2 level groups (20-40, 41-70, 71-145) mmhg. HCO3 mean 21.38 (9.7-34), groups (9.2-18, 18.1-25, 25.1-36). BE mean - 3.98 (-15.3 to +6), BE groups (-16 to -8, -7.9 to 6). HB mean 14.5 (7.3-24) g/dl. Post extubation ventilation mode and set are chosen by attending physician. Ventilation mode groups (Continuous positive airway pressure CPAP, Noninvasive NAVA ventilation “NIV NAVA”, non-invasive ventilation). PIP level mode 8 cm H2O. PIP groups (5-8, 9-30) cm H2O. FIO2 level mode 0.21, groups (0.21-0.30, 0.31-0.90). PEEP level (<6, 6-7, 8-10) cm H2O. Ventilation rate (15-30, 31-60)/min. Edi max mean 7 (2.8- 12) μV. Edi max mean 1.4 (0.2-5) μV. PH level mode 7.3, groups (6.9- 7.24, 7.25-7.34, 7.35-7.55). PCO2 level mode 42 mmhg, PCO2 groups (19-34, 35-54, 55-86) mmhg. PO2 level mode 40 (18-127) mmHG, groups (20-40, 41-70, 71-145) mmHG. HCO3 level groups (10-18, 18.1-25, 25.1-36) mEq/L. Each VLBW fails extubation and re-intubated has CXR to check ETT tip position. The major finding for all GA groups is ground glass appearance 60%, 51.6%, 87.5% for GA group (21+4- 26+6, 27- 29+6, 30-32). CXR at failure groups (nonground glass appearance, significant ground glass appearance). Times of extubation (1st, 2nd, 3rd). Extubation day (within 2days of life, within 7days of life, later). Air leak groups (No air leak, air leak syndrome till 14 days of life). Air leak syndrome include [PIE, Tension pneumothorax]. PDA (Tiny PDA and no treatment needed, Large significant PDA requiring treatment). IVH grade (No IVH, low grade 1&2, advanced grade 3&4). Continuous variables analysis is Mean and One-way ANOVA. We don’t have a non-parametric test that analyzes the effects of all predictors at once.
Results
The study evaluates 446 neonates <32 weeks GA, or < 1500g BWT. 132/446 are not fit for extubation trial till the end of 14 days of life.
1st trial extubation (till 14 days of life)
314/446 infant 1st extubated after assessment of clinical stability by attending physician. (before completing 14 days of life).
222/314 (70.7%) infant succeed at 1st trial extubation (success is defined by passing 5 days without re-intubation).
92/314 (29.29%) infant fail at 1st trial extubation (need re-intubation within 5 days post extubation).
2nd trial extubation (included only within 7 days of life to observe patients over the next 5 days)
We exclude 34/92 trial post 1st week.
15/92 infant are not fit for extubation till 14 days of life.
43/92 infant, 2nd trial extubation (within 7 days of life).
29/43 infant succeed 2nd trial extubation.
14/43 infant fail 2nd trial of extubation.
3rd trial extubation (only within 7 days to observe patients over the next 5 days)
We exclude 12 patients are not fit for extubation within the first 7 days of life.
3 infants, 3rd trial extubation within 1st week.
2 infants succeed 3rd trial extubation.
1 infant fails 3rd trial extubation.
Discussion
Our study is enlighted by the paper of Shih-Hsin Wang and his colleagues 2015, the only predictor that their study proved to be related to extubation failure is poor acid - base homeostasis 2 hours after extubation (pH < 7.3 and HCO3 < 18 mM/L) regardless of premature GA [12]. Our study is unique for the sample size and the analysis of subgroups GA. The following is discussion of results in Tables 1,2,3.
One dose of antenatal steroid is enough to statistically predict extubation success for GA (27- 29+6) weeks, and 2nd dose not add to the significance. Unrecorded cases may underestimate the effect of steroid (bias).
Adequately sized GA group (30-32) weeks, have moderate association with extubation success than small for GA. Male gender of GA (21+4-26+6) weeks is related to extubation failure, and no association for other GA groups.
Table 1:Results.
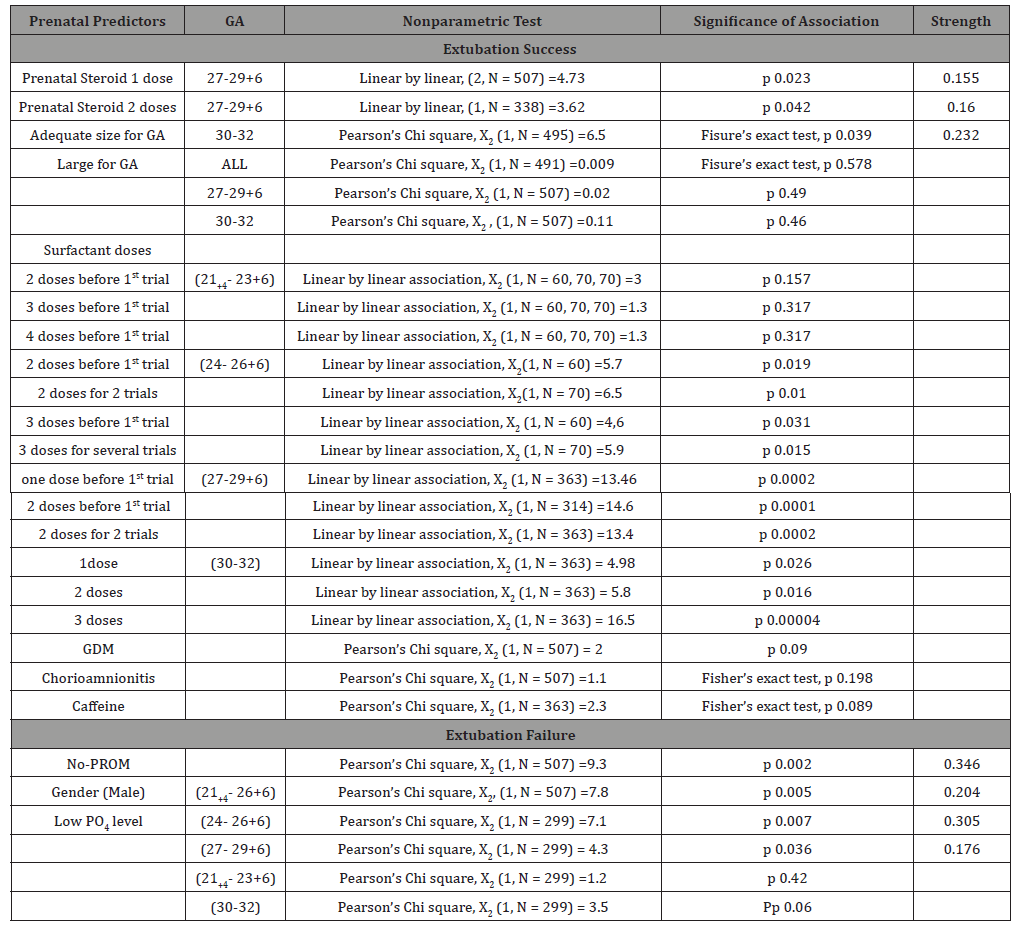
Table 2:Results.
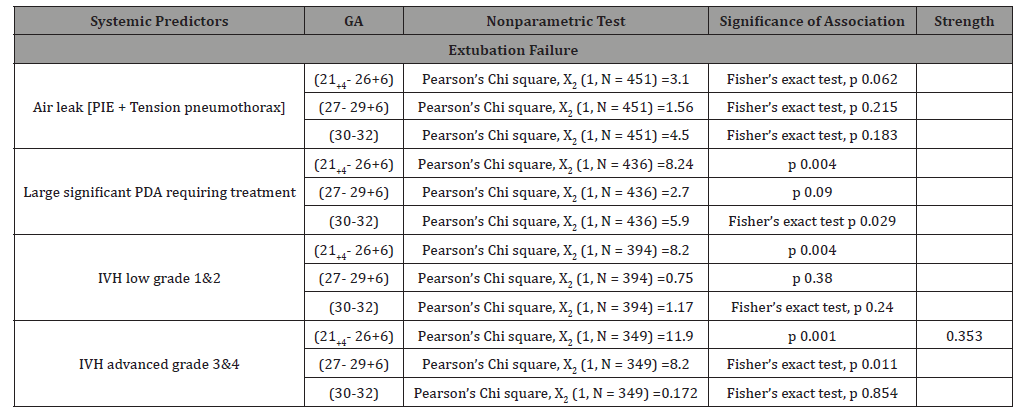
Table 3:Results.
GA group (21+4-23+6) w is 1 at canalicular stage of fetal lung development and has no surfactant production. All doses of surfactant are not enough to make a statistical difference in extubation trial. The underdeveloped lung is not fit for extubation trial, dependent on invasive ventilation and surfactant is not enough alone to be the treatment of choice to prevent extubation failure. GA group (24- 26+6) w is at saccular lung maturity stage. Surfactant is detected in fetal amniotic fluid at this GA, and 2 doses of surfactant are enough to compensate for surfactant deficiency and make a statistically significant association with extubation success. GA group (27- 29+6) w is at fetal saccular lung maturity stage with much surfactant production. One dose of surfactant is enough for extubation trial success, and further doses do not add to significance. GA group (30-32) w lung has well established surfactant production, and in case of deficiency, all accumulative surfactant doses are associated with extubation trial success. Extra factors causing surfactant deficiency in GA group (30-32) w, need to be studied further. Gestational diabetic status GDM, Chorioamnionitis are not related to extubation trial. Unknown cases may drift results and underestimate the infectious effect on extubation trial.
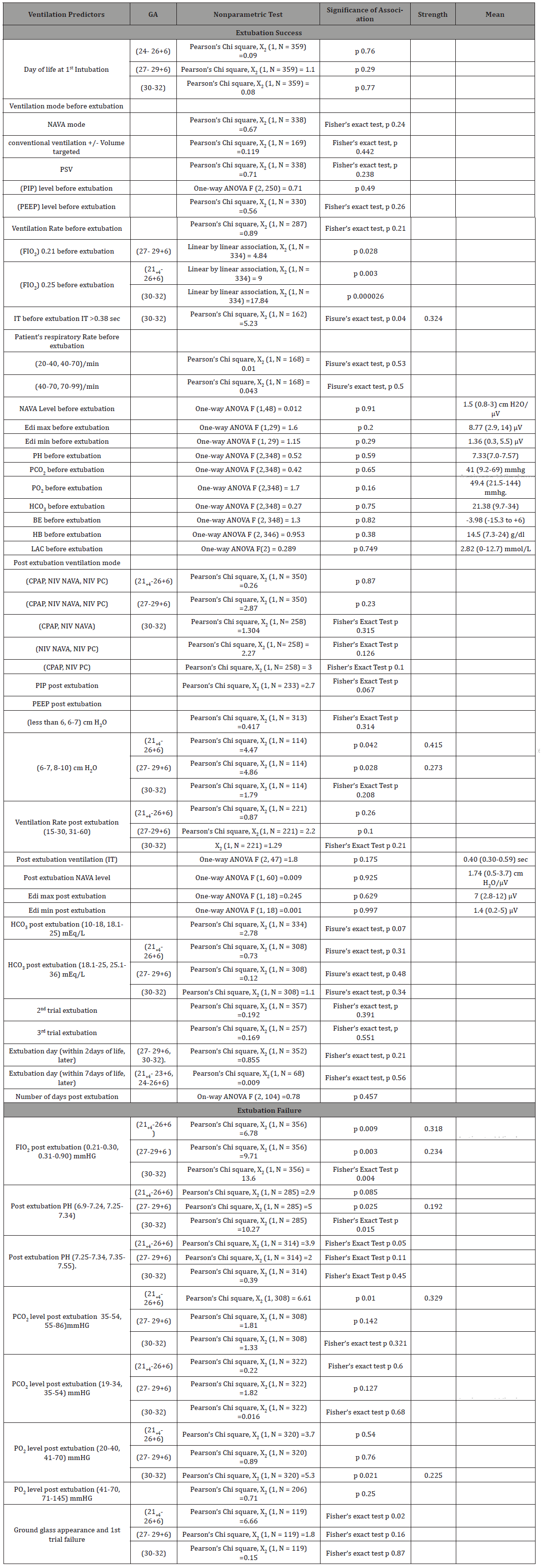
Pre-labor ruptured membrane (PROM) seems to be a predictor for extubation success, as NO PROM group is statistically associated with extubation trial failure for all GA groups. Caffeine treatment is not related to extubation trial for all GA groups. Low PO4 level at 2 weeks of life is strongly associated with extubation failure for GA (24- 26+6) weeks, mildly associated for GA (27- 29+6) weeks, but not for GA groups (21+4- 23+6, 30-32). Optimal PO4 supplement added to parenteral nutrition may add to extubation trial success. All GA groups (21+4- 23+6) w infants included are intubated at delivery room for respiratory failure. Analysis of day of 1st intubation (delivery room, later) is not visible, but not related to extubation trial for other GA groups. Ventilation Mode, PIP, PEEP, Rate before extubation are not related to extubation trial for all GA groups. Reducing the fraction of inspired oxygen (FIO2) to 0.21 before extubation associates extubation success trial for GA group (27- 29+6) w, while (0.25) is enough for success for GA group (21+4- 26+6, 30-32) w. This result is determined by bedside practice, and (0.21) is recommended as a guide.
Short Inspiratory time before extubation (IT) groups (0.2-0.33, 0.33-0.37) sec act the same and has no relation with extubation trial. Longer IT groups (0.38-0.4, 0.4-0.86) sec act the same and has no relation with extubation trial. Comparison between 1 (0.20-0.37, 0.38- 0.86) sec is significant for GA group (30-32) w. Inspiratory time before extubation (IT) (0.38-0.86) sec associates trial success for GA group (30-32) w. As the longer IT group (0.4-0.86) sec act the same as IT group (0.38-0.4) sec, and RDS management requires lower possible range of IT, we recommend starting with IT range (0.38-0.4) sec for safe ventilation and successful extubation trial. Patient’s respiratory rate before extubation (RR) is not related to extubation trial. Tachypneic VLBW may still succeed extubation trial, as well as variably slow breathing VLBW. Neutrally adjusted ventilatory assist NAVA Level, electrical activity of the diaphragm (Edi) (max & min) before extubation are not related to extubation trial. Blood gas PH, PCO2, PO2, HCO3, BE, HB, LAC before extubation are all not related to extubation trial.
Post extubation ventilation mode, PIP, Rate, IT, NAVA level, Edi (max & min) are not related to extubation trial. Low PEEP level (<6, 6-7) cm H2O post extubation is not related to 25 extubation trial for all GA groups. 26 For high PEEP level (6-7, 8-10) cm H2O post 27 extubation trial, extubation trial success is 28 related to PEEP level (6-7) cm H2O for GA 29 (21+4- 26+6, 27- 29+6) w, but not for GA group 30 (30-32) w. Ventilation FIO2 level > 0.30 post extubation associates extubation trial failure for all GA groups. Metabolic acidosis post extubation associates extubation failure for GA groups (27- 29+6, 30-32) w, but not for GA (21+4 -26+6) w. For GA (21+4 -26+6) w metabolic acidosis is somehow a characteristic feature and is not a good predictor for extubation failure. At this point our study differ in results from the study of Taiwan 2015, because in mixing all GA groups, metabolic acidosis is a predictor for failure for all GA groups. Metabolic alkalosis is not related to extubation trial for all GA.
High PCO2 level > 55 mmhg post extubation associates extubation trial failure for GA (21+4- 26+6). Physician decided to re-intubate VLBW (trial fail) selectively at this level of PCO2, and the analysis show no association between PIP, ventilation Rate and extubation trial, therefore giving a chance of ventilation set adjustment to reduce PCO2 level by increasing PIP or Rate of ventilation may not be helpful to turn the trial result into success for GA (21+4- 26+6). Low PCO2 is not related to extubation trial success.
Post extubation ventilation rate grouped into (15-30, 31-60)/ min. PCO2 post extubation and ventilation Rate are not related for all GA groups. One Way ANOVA F (2, 218) =1.59, p=0.206. At failure we grouped infants’ respiratory rate into (0-30, 31-60, 61- 100)/ min. PCO2 mean is not related by general linear model test to infant’s respiratory rate post extubation (failure group only) for all GA groups, and extra factors need to be studied further. One Way ANOVA F (2, 108) =0.39, p=0.677. No relation between high PCO2 and extubation trial for (27- 29+6, 30-32).
Low PO2 level post extubation is not related to the trial for GA groups (21+4- 26+6, 27- 29+6) w, but associate’s failure for GA (30- 32) w. High PO2 post extubation is not related to trial for all GA groups. Post extubation blood gas-HCO3 is not related to extubation trial.
Chest XR at 1st extubation failure, showing significant ground glass appearance is statistically significant for GA group (21+4- 26+6). We recommend a new study to determine the efficacy of performing CXR before extubation trial to guide extubation decision for GA group (21+4- 26+6). CXR at 2nd extubation failure showing Non-ground glass appearance for GA group (21+4- 26+6) is statistically significant. At 2nd trial failure for GA group (21+4- 26+6), CXR cleared of ground glass appearance but trial may still fail for other reasons. CXR at failure is not related to extubation trial for further mature GA groups (27- 29+6, 30-32). Times of extubation trial (1st, 2nd 3rd are not related to extubation trial results.
1st extubation within 2days of life is not related to trial results for GA groups (27- 29+6, 30-32) w. 1st extubation within 7days of life is not related to trial results for GA groups (21+4- 23+6, 24- 26+6) w. GA group do not predict day of failure post extubation trial. On-way ANOVA F(2, 104) =0.78, p=0.457. Air leak syndrome is not related to extubation trial for all GA groups. Patent Ductus Arteriosus (PDA) is not related to extubation trial for GA groups (27- 29+6) w. Large significant PDA requiring treatment is associated with extubation failure for GA group (21+4- 26+6, 30-32) w. Bedside practice may drift results. Low grade IVH (1&2) associates extubation failure for GA group (21+4-26+6) w, but not related for GA groups (27- 29+6, 30-32) w. Advanced IVH grade (3&4) associates extubation failure for GA groups (21+4- 26+6, 27- 29+6) w, but is not related for GA (30-32) w.
Do Times Extubation Increase IVH Rate?
low grade IVH is not related to extubation times for all GA groups. Pearson’s Chi square, X2 (1, N = 302) =0.385, Fisher’s exact test, p 0.37. Advanced grade IVH is not related to extubation times for all GA groups. Pearson’s Chi square, X2 (1, N = 340) =0.12, 3.4, 1.51. Fisher’s exact test, p 0.49, 0.06, 0.23.
Conclusion
Predictors of extubation trial failure are different between GA groups. Associated risk factors may guide physician to predict the result of extubation trial and reduce exposure to trial failure. Accurate documentation of antenatal history is critical for statistical significance of association. Follow up research studies on larger sample size are recommended to analyze secondary predictors.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, et al. (2010) European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants - 2010 update. Neonatology 97(4): 402-417.
- Roberts D, Brown J, Medley N, Dalziel SR (2006) Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev (3): CD004454.
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, et al. (2010) Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126(3): 443-456.
- Kalikkot Thekkeveedu R, Guaman MC, Shivanna B (2017) Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir Med 132: 170-177.
- Strong RM, Passy V (1977) Endotracheal intubation. Complications in neonates. Arch Otolaryngol 103(6): 329-335.
- Stein H, Firestone K, Rimensberger PC (2012) Synchronized Mechanical Ventilation Using Electrical Activity of the Diaphragm in Neonates. Clin Perinatol 39(3): 525-542.
- Stefanescu BM, Murphy WP, Hansell BJ, Fuloria M, Morgan TM, et al. (2003) A randomized, controlled trial comparing two different continuous positive airway pressure systems for the successful extubation of extremely low birth weight infants. Pediatrics 112(5): 1031-1038.
- Baisch SD, Wheeler WB, Kurachek SC, Cornfield DN (2005) Extubation failure in pediatric intensive care incidence and outcomes. Pediatr Crit Care Med 6(3): 312-318.
- Epstein SK, Ciubotaru RL, Wong JB (1997) Effect of failed extubation on the outcome of mechanical ventilation. Chest 112(1): 186-192.
- Narchi H, Chedid F (2015) Neurally adjusted ventilator assist in very low birth weight infants: Current status. World J Methodol 5(2): 62-67.
- Colaizy TT, Kummet GJ, Kummet CM, Klein JM (2017) Klein Noninvasive Neurally Adjusted Ventilatory Assist in Premature Infants Postextubation. Am J Perinatol 34(06): 593-598.
- Wang SH, Liou JY, Chen CY, Chou HC, Hsieh WS, et al. (2017) Risk Factors for Extubation Failure in Extremely Low Birth Weight Infants. Pediatr Neonatol 58(2): 145-150.
-
Masoud Rasha, Lanqawi Noura, Respiratory Support Adequacy for Very Low Birth Weight Infants Post Extubation. Glob J of Ped & Neonatol Car. 2(2): 2020. GJPNC.MS.ID.000531.
Post extubation, Low birth weight infants, ELBW, Extubation failure, Risk factor, Bronchopulmonary dysplasia, Ventilation, NAVA, Extubation trial, Ventilation mode
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






