 Research Article
Research Article
Prevalence of Different Types of Leukemia and Associated Factors among Children with Leukemia in Children’s Cancer Units at Al-Kuwait Hospital, Sana’a City: A Cross-Sectional Study
Monya Abdullah Yahya El-Zine2, Abdulrahman M Alhadi1, Abdulrahman A Ishak1 and Hassan A Al- Shamahy3*
1Department of Pediatrics, Sana’a University, Republic of Yemen.
2Department of Histopathology, Sana’a University, Republic of Yemen
3Medical Microbiology and Clinical Immunology Department, Faculty of Medicine and Health Sciences, Sana’a University, Republic of Yemen.
Hassan A Al-Shamahy, Medical Microbiology and Clinical Immunology Department, Faculty of Medicine and Health Sciences, Sana’a University, P.O. Box 775 Sana’a, Yemen.
Received Date: April 24, 2021; Published Date: June 14, 2021
Abstract
Background and aims: Leukemia is a heterogeneous group of haematological disorders that is made up of several diverse and biologically distinct subgroups. Leukemia is the eleventh and tenth most common cause of cancer morbidity and mortality worldwide, respectively. There are insufficient data on the prevalence and associated factors of leukemia in Yemen, particularly in the study area. This cross-sectional study aims to determine the prevalence of different types of leukemia and associated factors among children with leukemia in the pediatric cancer units of Al- Kuwait Hospital, Sana’a City. Patients and method: A cross-sectional study was conducted on children with leukemia who were treated selectively in the pediatric leukemia units of Kuwait University Hospital in Sana’a. Group diagnostics and histopathological diagnoses were formed in line with the French, American and British classifications of leukemia in children in the pediatric leukemia units, over a period of 5 years from January 1, 2014 to December 31, 2018. Factors associated with become infected with leukemia that were studied included ages, sex, and outcomes. The association of death and recovery with different age groups and leukemia types was also studied through rates and calculation of OR, CI, X2 and p values through probability tables (2x2 tables). Results: 244 leukemia patients were diagnosed, treated and followed up; there was association of leukemia with younger age group; 50% were in the age group 1-5 years and with mean ± SD age= 6.44 ± 3.7 years. There was significant association with male (66.7%). There was association between high mortality and the 6-10 year age group [(8/78; 10.2%), with OR = 2.6, p = 0.060], and with the AML [(4/38; 10.5%), OR = 2.1)]. Considering, the cure rates association with ages, roughly there were similar cure rates occurred the different age groups. Also, there was high cure rate occurred in the JCM (2/3; 66%), with OR = 2.9. and with the CML (7/11; 63.6%; OR = 2.60. Conclusion: In the current study there was an association between leukemia and younger age group, with males. There was an association between high mortality and the 6-10 year age group, with AML. Also, there was no association between ages and cure rate, but a high cure rate occurred with JCM and CML. More comprehensive investigations of relevant factors and predictors are needed with more modern diagnostic methods and investigate correlation factors with the treatment protocols used.
Keywords:Childhood leukemia; Associated factors; Odds ratio (OR); Death rate; Cure rate; Yemen
Introduction
Leukemia is the most common type of cancer in childhood, accounting for 25 percent of all cancers that occur before the age of 20. There are two main types of childhood leukemia - acute lymphocytic leukemia (ALL), which accounts for about three quarters of leukemia, and acute myeloid leukemia (AML) accounting for most of the rest of the leukemia cases. ALL is a disease that affects about three quarters of leukemia. The hematopoietic tissue in the bone marrow is characterized by the overproduction of immature lymphocytes (a type of white blood cell). ALL occurs at all ages, from birth to adulthood, but the incidence peaks between 2 and 6 years of age. In the United States, there is a majority of white and male children and young adults with ALL. Improvements in treatment have led to remarkable gains in survival, estimated at 79 percent at 5 years. Acute myeloid leukemia is a cancer of the myeloid white blood cell line that occurs at all ages from childhood. The outcome for AML is poorer than for ALL, with a 5-year survival rate of 41 percent [1,2]. The precise cause of leukemia is not up till now obvious. Nevertheless, a lot of factors, mainly genetics, genetic mutations, epigenetic lesions, ionizing radiation, other chemical and occupational contacts, curative drugs, smoking and some viral agents, have been concerned in the development of leukemia [3-8]. Commonly 2 types of classification systems are used for leukemia: (the Franco-American and British classification system (FAB), which relies on morphology and cytochemical staining to identify specific types of leukemia, and the World Health Organization (WHO) that reviews classification information, cytomorphology, cell chemistry, immune profiling, cytogenetics and clinical features to identify and classify clinically significant disease entities [9,10]. Lymphoma malignancies correspond to a heterogeneous group of illnesses separated into four classes established on tumor cell maturity and disease distribution such as acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), lymphoma malignancies, plasma cell tumors and hairy cell leukemia [11].
Globally Leukemia involved 200,676 males and 151,289 females (with ASR 5.6 and 3.6, respectively). There were 46,449 males and 35,880 females diagnosed in 2012 with leukemia in Europe. In Australia, Asia and the USA about 233,451 residents were diagnosed with leukemia by 2017. In contrast, in Africa by 2012, it has a rate of incidence equal to 23,928 cases (ASR 3.0 per 100,000) [12-14]. Leukemia was the most common type of cancer among children (including 29% of 3,707 cancer cases in children, and acute leukemia counting 89% (91% of which were ALL and 9% AML) of all cases of leukemia in children [15].
In developing countries, the influence of leukemia is massive attributable to premature death of children, loss of parents, failure of productivity due to disability, and high medical costs affecting the social, economic and health well-being of the population [16- 18]. While leukemia is treated very well in the developed world, there is little evidence of the current state of the disease in Yemen in general and in the study area in particular. On the other hand, in Yemen as is the case in most Arab countries, there are few specialized epidemiological records devoted to this area, and for this reason it is important to encourage, update, build and continue to present studies on childhood leukemia with the goal of achieving greater impact on public health, with early diagnosis and appropriate treatment aimed at enhancing survival and minimizing potential consequences. According to the limited Yemeni Cancer Studies, the most common types of cancer among Yemeni children and adults were leukemia (33.1%), lymphoma (31.5%), central nervous system tumors (7.2%), and bone tumors (5.2%) [19- 21]. This cross-sectional study aims to determine the prevalence of different types of leukemia and the associated factors among children with leukemia in the pediatric cancer units of Al-Kuwait Hospital, Sana’a City.
Patients and Method
A cross-sectional study was conducted on children with leukemia who were treated selectively in the pediatric leukemia units of Al-Kuwait University Hospital in Sana’a. Group diagnostics and histopathological diagnoses were formed in line with the French, American and British classifications of leukemia in children in the pediatric leukemia units, over a period of 5 years from January 1, 2014 to December 31, 2018. Factors associated with become infected with leukemia that were studied included ages, sex, and outcomes. The association of death and recovery with different age groups and leukemia types was also studied through rates and calculation of OR, CI, X2 and p values through probability tables (2x2 tables).
Statistical Analysis
Data were recorded using appropriate descriptive statistics (including frequency, mean, and standard deviation). The odds ratio (OR) was used to determine the strength of the association between two events, such as leukemia, age, gender, and residence. The association between death, age, and type of leukemia. In addition to calculating the relationship between cure, age of patients, and types of leukemia. The two events in the current study were independent if and only if OR was equal to 1. For sample constraints of odds ratio in small numbers (less than 5), Fisher’s exact test was used as an alternative estimator for the association between events in the current study.
Ethical Approval
Ethical approval was obtained from the Medical Research & Ethics Committee of the Faculty of Medicine and Health Sciences, Sana’a University. All data, including patient identification were kept confidential.
Results
Table 1 shows the age and gender distribution of children with childhood leukemia in Sana’a, Yemen. The mean ± SD age of all cases was 6.44 ± 3.7 years. Most of the cases were in the age group 1-5 years (50%), followed by the age group 6-10 years (32.1%), while only 17.9% of the cases were in the age group 11-15 years (disease decreases with increasing age). As for gender, most of the cases were males (66.7%), while the percentage of females was 33.3% (male to female ratio = 2-1). Table 2 shows leukemia outcomes among children suffering from childhood leukemia in Sana’a, Yemen. The cure rate was 40.7% while the death rate was 15 cases (6.2%), all of them male (male mortality rate = 9.3%). The relapse rate was 2%. The rest of the cases were in maintenance therapy (31.5%), induction therapy (15.4%), and consolidation (post-remission therapy) for 4.3% of cases. Table 3 shows the age-group association of death among children with childhood leukemia in Sana’a, Yemen. The highest mortality occurred in the 6-10 year age group (8/78; 10.2%), with an associated OR = 2.6, CI = 1-7.4, X2 = 3.4, p = 0.06. However, the low mortality rate was 3.3% in the 1-5 year group, without association (OR = 0.34, p = 0.06). Also, in the 11--15 yearold group, the death rate was 6.8% roughly similar to the overall death rate (6.1%).
Table 1:Age and gender distribution of children with childhood leukemia in Sana’a, Yemen.

Table 2:Leukemia outcomes among children suffering from childhood leukemia in Sana’a, Yemen.
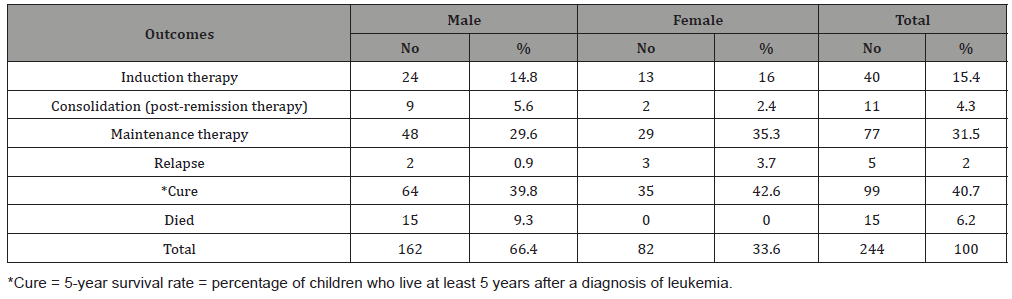
Table 3:The association of death with age groups among children suffering from childhood leukemia in Sana’a, Yemen.
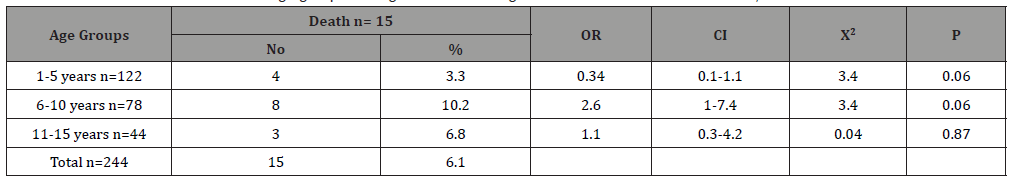
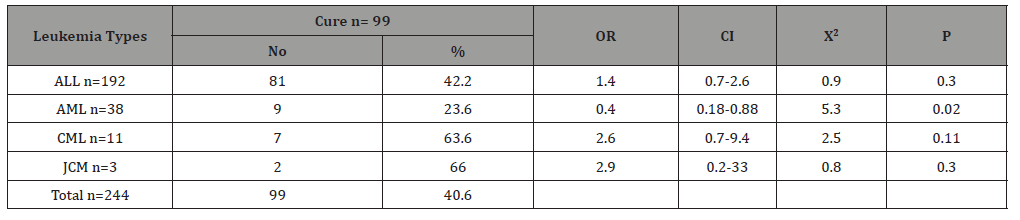
Table 4 shows the type of leukemia association of death among children with childhood leukemia in Sana’a, Yemen. Acute lymphoblastic leukemia was the most common, accounting for 78.7% of the total, while the other types were less common, with acute myelogenous leukemia count of 15.6%, chronic myelogenous leukemia at 4.5% and Juvenile myelomonocytic leukemia only at 1.2. %. The highest mortality occurred in the AML (4/38; 10.5%), with an associated OR = 2.1, CI = 0.6 – 6.9, X2 = 1.5, p = 0.22. However, the mortality rate was 5.7% in the ALL patients, without association (OR = 0.72, p = 0.6).There was no death occurred in CML and JCM cases (0%). Table 5 shows the age-group association of cure among children with childhood leukemia in Sana’a, Yemen. The cure rates were roughly similar in the different age groups. It is ranged from 38.5% in 1-5 year group to 43.2% in 11-15 year group. Table 6 shows the type of leukemia association of cure among children with childhood leukemia in Sana’a, Yemen. The highest cure rate occurred in the JCM (2/3; 66%), with an associated OR = 2.9, CI = 0.2 – 33, X2 = 0.8, p = 0.3. The second high cure rate occurred in the CML (7/11; 63.6%), with an associated OR = 2.6, CI = 0.7 – 9.4, X2 = 2.5, p = 0.11. However, the cure rate was 23.6% in the AML patients, without association (OR = 0.4, p = 0.02). The cure rate was 42.2% in the ALL patients, with association (OR = 1.4, p = 0.3).
Table 4:The prevalence and association of death with type of leukemia among children suffering from childhood leukemia in Sana’a, Yemen.
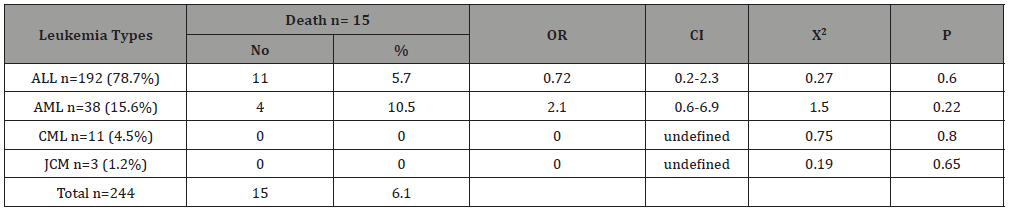
Table 5:The association of cure with age groups among children suffering from childhood leukemia in Sana’a, Yemen.
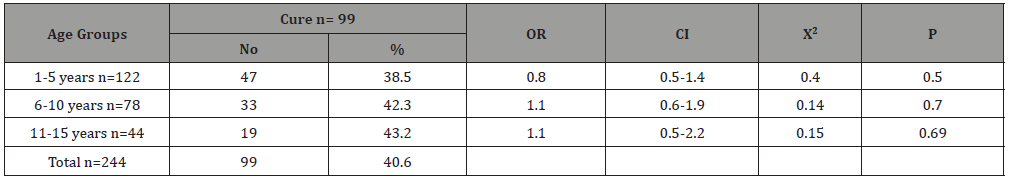
Table 6:The association of cure with type of leukemia among children suffering from childhood leukemia in Sana’a, Yemen.
Discussion
Information of the prevalence of leukemia in a population may envisage pathogenic hypotheses for disease control and aid in the effective management of leukemia and other malignant hematomas. In developing countries, and especially in Yemen, there is little information about the burden and patterns of haematological malignancies, especially leukemia. In the current study, with regard to gender, most of the cases were male (66.7%) while the percentage of females was 33.3% (male to female ratio = 2:1). This finding differs from that reported in Africa where the ratio of males to females is roughly equal, although females dominate slightly (1: 1.06) [22], but similar to that reported from the United States where the American Cancer Society estimates for leukemia in 2021, about 5,690 new cases, 3,000 in males and 2,690 in females [23]. The current results of different leukemia prevalence rates among gender are in line with the facts that the prevalence of leukemia must be varies according to gender due to the biological factors [11,15,24,25].
Leukemia may appear at all ages, from newborns to the elderly, but the distinctive forms have different age distributions [26]. In the current study, the mean age of ± SD for all cases was 6.44 ± 3.7 years and most of the cases were in the age group 1-5 years (50%), followed by the age group 6-10 years (32.1%), while only 17.9% of the cases were in the age group 11-15 years (Table 1). This is similar to what has been reported elsewhere for pediatric leukemia where the mean age ± SD of pediatric leukemia cases was 6.0 years with a peak incidence at 6-10 years [2,12,14]. This differs from the leukemia hypothesis with age in which older children may develop leukemia more frequently than younger children due to advancing age, as many environmental exposures to carcinogens, irradiation, and malignant mutations due to clonal expansion occur more often [27,28]. However, most of the younger children in the current study could be explained by the fact that prenatal and early life exposure is thought to be important determinants of childhood leukemia. Several mechanisms have been identified through which external and internal factors can influence the risk of developing leukemia in children. Exposure to a carcinogen or toxic substance early in a female’s life may cause permanent damage. Since no new oocytes are formed after birth and their maturation begins during pregnancy, the exposure that occurs during this critical time can be of great importance. During pregnancy, exposure to agents such as ionizing radiation may act directly while others may act indirectly by transporting the placenta. On the other hand, offspring may be exposed after birth to environmental exposure, either directly or indirectly [29]. Since most of the children are from rural areas, they may have been exposed to various environmental exposures during their stay with their parents who are farmers.
Environmental factors, even though not well articulated, influence the chance of developing leukemia. In this study, the highest proportion of 68.9% out of the total of 244 patients diagnosed with leukemia was observed among rural residents (p < 0.05). In Yemen, rural residents’ lifestyle is based on agricultural activities such as farming and plantations agriculture; especially Gat, fruits and vegetables plantation are the major practice around the study area, thus this may lead to the repeated use of chemicals such as pesticides, herbicides, and fertilizers for agricultural activities which will result in genetic mutations conferring leukemia [30].
Leukemia types were determined using the FAB classification method [10,11], Wright-stained morphological examination, and cytochemical staining with Sudan black B staining to differentiate the cell lineage. In this study, acute lymphocytic leukemia was the most common, accounting for 78.7% of the total, while the other types were less common, with acute myelogenous leukemia count of 15.6%, chronic myeloid leukemia 4.5%, and juvenile myeloid leukemia at only 1.2%. This result was consistent with results from Ethiopia, Nepal, and Pakistan [22,31], while it was contradictory with a study from Albania [32]. In the current study, the highest mortality occurred in the 6-10 year age group (8/78; 10.2%), with an associated OR = 2.6, CI = 1-7.4, X2 = 3.4, p = 0.06. However, the low mortality rate was 3.3% in the 1-5 year group, without correlation (OR = 0.34, p = 0.06). These results are consistent with the American Cancer Society’s fact that most cases of leukemia occur in young children, but most deaths occur in older children. Very young children may perform better than older children due to differences in the nature of leukemia in children, differences in treatment (often young children’s bodies can handle aggressive treatment better than older children), or a combination of these [23]. The highest mortality occurred in AML (4/38; 10.5%), cure rate = 23.6%. However, the mortality rate was 5.7% in ALL patients, with cure rate = 42.2%. These results are in line with findings by the Leukemia and Lymphoma Society of the USA where the 5-year survival rate for children and adolescents under the age of 15 years diagnosed with ALL was 91.8% in the United States between 2007 and 2013. While the survival rate for children under 15 years of age with AML was only 66.4% for the same period [33].
Conclusion
ALL is the most common type of leukemia in Sana’a city; and males and young children are affected the most by leukemia. In the current study there was an association between leukemia and younger age group, with males. There was an association between high mortality and the 6-10 year age group, with AML. Also, there was no association between ages and cure rate, but a high cure rate occurred with JCM and CML. More comprehensive investigations of relevant factors and predictors are needed with more modern diagnostic methods and investigate correlation factors with the treatment protocols used.
Acknowledgment
Thanks to the Pediatric Leukemia Unit at Kuwait Hospital, specifically to Dr. Abdulrahman Al-Hadi, a human doctor who spends his life, knowledge and time for children who suffer from this malignant disease.
Conflict Of Interest
No conflict of interest associated with this work.
References
- Berkus MD, Langer O, Samuelloff A, Zenakis EM, Field NT, et al. (1994) Meconium stained amniotic fluid: Increased risk for adverse neonatal outcome. Obstet Gynecol 84: 115-120.
- Nathan L, Lenevo KJ, Camody TJ, Kelly MA, Sherman ML (1994) Meconium: a 1990s perspective on an obstetric hazard. Obstet Gynecol 83: 329-332.
- Ahanya SN, Lakshmanan J, Morgan BL, Ross MG (2005) Meconium passage in utero: mechanisms, consequences, and management. Obstet Gynecol Surv 60: 45-56.
- Wiswell TE (2001) Handling the meconium stained infant. Semin Neonatol 6(3): 225-231.
- Wiswell TE, Henley MA (1992) Intratracheal suctioning, systemic infection and the meconium aspiration syndrome. Pediatrics 89: 203-206.
- Cleary GM, Wiswell TE (1998) Meconium-stained amniotic fluid and the meconium aspiration syndrome - An update. Pediatr Clin North Am 45: 511-529.
- Maymon E, Chaim W, Furman B, Ghezzi F, ShohamVardi I, et al. (1998) Meconium stained amniotic fluid in very low risk pregnancies at term gestation. Eur J Obstet Gynecol Reprod Biol 80: 169-173.
- Wiswell TE, Bent RC (1993) Meconium staining and the meconium aspiration syndrome. Unresolved issues. Pediatr Clin North Am 40: 955-981.
- Kligner MC, Kruse J (1999) Meconium aspiration syndrome: Pathophysiology and prevention. J Am Board Fam Pract 12: 450-466.
- Romero R, Hanaoka S, Mazor M, Athanassiadis AP, Callahan R, et al. (1991) Meconium-stained amniotic fluid: a risk factor for microbial invasion of the amniotic cavity. Am J of Obstet Gynecol 164(3): 859-862.
- Shukla OS, Swapna ST (2019) Study of risk factors, clinical profile, and outcome in meconium-stained deliveries. Indian J Child Health 6(5): 213-216.
- Divia A (2018) Study on Risk Factors and Perinatal Outcome in Meconium Stained Liquor, Dissertation pp. 64-70.
- Gupta V, Bhatia BD, Mishra OP (1996) Meconium stained amniotic fluid: antenatal, intrapartum and neonatal attributes. Indian Pediatrics 33(4): 293-297
- Patil KP, Swamy MK, Samatha K (2006) A one year cross sectional study of management practices of meconium stained amniotic fluid and perinatal outcome. Obstet Gynecol India 56: 128-130.
- Bhatia BD, Gupta V, Dey PK (1996) Meconium aspiration syndrome: current concepts. Indian J Matern Child Health 7(1): 1-7.
- Khatun M (2005) Meconium Staining liquor and its correlative with fetal outcome within seven days of birth in Dhaka Medical College. Dissertation. Bangladesh College of Physicians and Surgeons pp. 39-43.
-
Monya Abdullah Yahya El-Zine, Abdulrahman M Alhadi, Abdulrahman A Ishak, Hassan A Al-Shamahy. Prevalence of Different Types of Leukemia and Associated Factors among Children with Leukemia in Children’s Cancer Units at Al-Kuwait Hospital, Sana’a City: A Cross- Sectional Study. Glob J of Ped & Neonatol Car. 3(4): 2021. GJPNC.MS.ID.000569.
Young hockey players, Injuries, Orthopedic examinations, Medical care, Children, Sports, Disorders, Limb, Fractures, Treatment
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






