 Research Article
Research Article
Immunoglobulin Levels (IgG, IgM And IgA): Normal Values for Healthy Infants and Children in Sana’a City- Yemen
Mogahid YH Nassar1,2, Mona Mohamed Salah3, Hassan A Al Shamahy3*, Rahma Taher Farea AL Magrami3 and Khaled A Al Moyed3
1Department of Clinical Pathology, University of Science and Technology, Sana’a, Yemen
2Laboratory Department, University of Science and Technology Hospital, Sana’a, Yemen
3Department of Microbiology, Faculty of Medicine and Health Sciences, Sana’a University, Sana’a, Yemen
Hassan A Al Shamahy, Department of Microbiology, Faculty of Medicine and Health Sciences, Sana’a University, Sana’a, Yemen.
Received Date: December 08, 2019; Published Date: December 16, 2019
Abstract
Background: Ig level assessment is often used in the diagnosis and follow up of immunodeficiency, as well as in studies investigate the prevalence of low serum levels in specific diseases.
Objectives: The main objective of this study was to determine the level of immunoglobulin in healthy infants and children in Yemen, to establish a national standard for immunoglobulin level among healthy children based on local data.
Methods:m Immunoglobulin concentration was estimated in 448 healthy children of both sexes of different age groups in Sana’a city. IgG, IgM and IgA serum immunoglobulins were determined by the commercial immunoturbidimetry method. The mean Levels ±SD, 95%CI, minimum, maximum and mode of immunoglobulins (Igs) were calculated and cross tabulation of significant variations were obtained by χ2 and p value.
Results: Four-point two percentage of children showed a high level of IgG, 78.6 % within normal level, and 17.4 % showed a low level. There was no one of tested children showed a high level of IgM, and 94.2 % of them were within normal level, and only 5.8% with low level. Also, 2.2 % showed high level of IgA, 57.6% within normal level and 40.2 % with a low level. The mean level of IgG for both sexes was 910±424mg/dl, while for males was 912±452mg/dl, and for females was 902±404mg/dl. The total mean level of IgM was 96.7±50mg/dl, for males was 96±56mg/dl, and for females was 97±41mg/dl and the variation between sexes was non-significant. The total mean level of IgA was 105±79mg/dl, for males was 98±76mg/dl, and for females was 112±82mg/dl, but the variation was non-significant. The mean values level of all immunoglobulins tested in this study were well correlated with age, where level increase with increasing age.
Conclusion: The study has provided useful information about the normal level of serum immunoglobulins concentration in infants and children, and the results can be taken as a reference values for Yemen. The serum IgG, IgM and IgA levels in most healthy children in our study were within normal range of the international standard, with mean value equal to that reported worldwide.
Keywords: Immunoglobulin; IgG; IgM; IgA; Normal Values; Infants; Children; Yemen
Introduction
Activated B cells differentiate into antibody-producing cells called plasma cells that secrete dissolved antibodies or memory cells that remain in the body for years afterwards in order to allow the immune system to remember the antigen and respond faster at future exposure. Immunoglobulin concentrations (IgG, IgM, and IgA) in sera for healthy children and infants in different age groups were measured and reported in several studies. It was believed to be very diverse among different nations due to environmental and genetic factors [1]. In Yemen, there was no study discussing this issue, and this is the first study of this type while other research studies have been conducted in Yemen to study HLA, immune status against bacterial and viral infections recently [2-6]. There is an important reason to know the concentration of immunoglobulin among children in Yemen as doctors need a precise age-normal range and ethnic-normal range of Igs, so they can diagnose primary immunodeficiency, some autoimmune disorders, HIV Syndromes and various isolated clinical conditions [1,7].
Antibody deficiency is the most common category of primary immunodeficiency for more than 50% of all primary immunodeficiency diseases (PID) [8] Severe antibody deficiency disease begins in childhood, so early diagnosis and treatment are key to improving better prognosis [9]. Blood immunoglobulin concentrations (IgG, IgA, IgM, IgG subclasses) are used to evaluate the immune status and diagnose PID [10]. A new classification of PID has been identified, [11,12], so age-specific immunoglobulin values are very important [11,12]. The main objective of this study was to determine the level of immunoglobulin in healthy infants and children in Yemen, to establish a national standard for immunoglobulin level among healthy children based on local data.
Material and Methods
Study population and sample size
Accordingly, a sample size of 448 subjects was calculated, this was selected by a systematic random method. First, all maternity centers (with children aged 1 month to 5 years), all schools and nurseries with children aged 5 to 15 years in Sana’a city (270 locations) were listed, then by simple random selection, 4 maternity centers, 8 schools and nurseries (4 female and 4 male); were selected. Subsequently, for schools and nurseries using a stratified sampling method, classes were selected by age to cover all relevant age groups (5 to 15 years); finally, each fifth child attending health centers and / or in the selected class list was selected. About 20% of male children and 17% of female children refused to donate blood or their parents were replaced by another cooperative child. All children were asymptomatic with natural immune systems while participating in the study. Successive blood samples were obtained from 448 healthy children. All individuals do not complain of any clinical symptoms and do not have any infection in the sampling period (or in the recent past).
Laboratory works
5 ml of venous blood were with drowning of each individual and allowed to clot, or with drowning in heparin tubes or EDTA tubes. Serum or plasma was stored at -20° C or tested immediately after collection and separation for total immunoglobulin (IgG, IgM and IgA) by immunoturbidimetry.
Determination of IgG, IgM, and IgA
Immunoglobulin G, M, and A precipitate in the presence of goat antihuman immunoglobulin G, M, and A antibodies. The light scattering of the antigen – antibody complexes is proportional to the immunoglobulins concentration and can be measured by turbidimetry (the kits were supplied by Biosystems.S.A.Company, Spain). The readings were taken at 540 nm. The results were calculated against the calibration curves and the results were obtained in mg/dl.
Data analysis
Basic Descriptive statistics, including means, standards deviations, modes, percentage and 95% CI of the mean were used to characterize the study populations. Associations between variables were measured using chi-square (χ2) or student’s t test. Statistical significance was achieved when p<0.05. Statistical analysis was performed by using Epi Info version 6- CDC, Atlanta USA Software.
Results
The study results illustrated in 8 tables. Four hundred and forty eight apparently healthy infants and children were involved in this study 260 were males (58%) and 188 were females (42%), their ages ranged from 2 months to - 15 years, with mean ages equal to 6.6 years, SD±5.1 years, median=5 years and mode =4 years (Table 1). Table 2 shows the IgG level of immunoglobulin for healthy children tested according to international standards, as 4.2% of children showed a high level of IgG>1660 mg/dl, 78.6% were at the normal level (600 mg/dl / - 1660 mg/dl ), and 17.4% showed a low level of IgG (less than 600 mg/dl). Table 3 shows the level of immunoglobulin M (IgM) for healthy children tested according to international standards. None of the children tested showed a high level of IgM, 94.2% of them were within the international normal level (40mg/dl- 120mg/dl) and only 5.8% showed a low level of IgM (<40 mg/dl). Table 4 shows the level of IgA immunoglobulin for healthy children tested according to international standards, 2.2% showed a high IgA level (>400 mg/dl), 57.6% was within the normal level (70 mg/dl - 400 mg/dl) and 40.2% showed low level% (<70 mg/dl). Table 5 shows the age-related serum IgG level (mg/ dl) with 95% CI levels in healthy children, and the crude mean level of IgG was 910±424 mg/dl, with 95% CI equal to 870-950 mg/dl. There was an increase in other values of IgG as age increased. Table 6 shows the age-related blood level of IgM (mg/dl) with 95% CI in healthy children, the mean level of IgM was 96.7±50 mg/dl, and other IgM values increased with increasing age. Table 7 shows the age-related blood level of IgA (mg/dl) with 95% levels in healthy children, the average level of IgA was 105±79 mg/dl, and other means and values of IgA were not statistically different with age. Table 8 shows the mean serum levels of IgG, IgM and IgA and gender comparison, and there was no statistical difference in the different immunoglobulin between male and female.
Table 1:The age and sex distribution of infants and children tested for serum immunoglobulin.
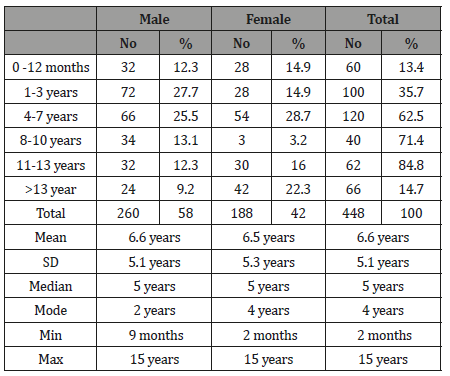
Table 2:The level of immunoglobulin IgG of tested healthy children according to the international standard.
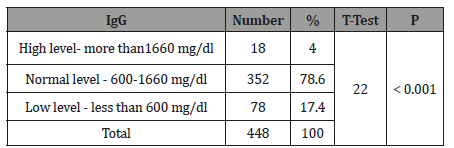
Table 3:The Level of Immunoglobulin M (IgM) of tested healthy children according to the international standard.
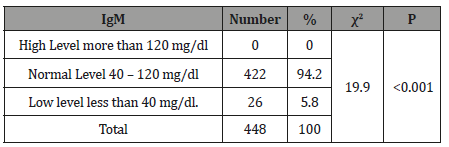
Table 4:The Level of Immunoglobulin IgA of tested healthy children according to the international standard.
Table 5:Age- related serum IgG level (mg/dl) with 95% CI levels in healthy children.
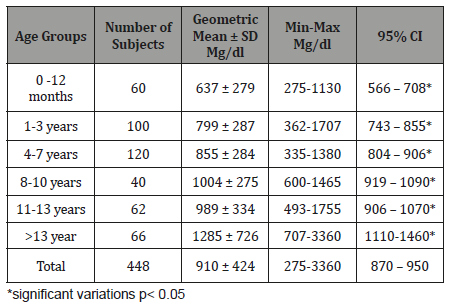
Table 6:Age related Serum IgM level (mg/dl) with 95% CI in healthy children.
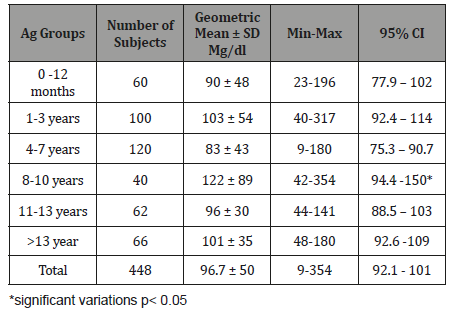
Table 7:Age related Serum IgA level (mg/dl) with 95% levels in healthy children.
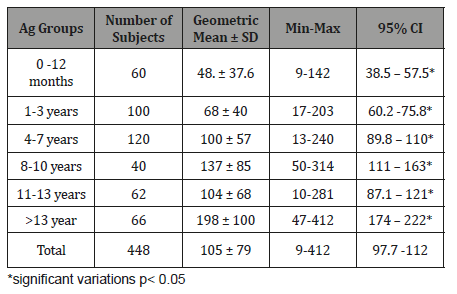
Table 8:Mean Serum Immunoglobulin levels and comparison between the two sexes (mg/dl).
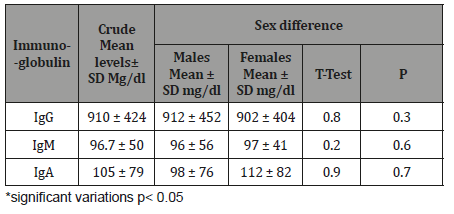
Discussion
The first objective of this study was to determine the level of immunoglobulin in the circulation of children. IgG is the predominant antibody that is synthesized in a secondary antibody response. IgG is an exclusive antioxidant antibody. Both the intravascular and extra vascular pools contain equal amounts of IgG. In our study, 78.6% of all children tested were at the normal level of IgG according to international standards (Table 2). However, 4% of the children tested showed a high level of IgG in the blood, and 17.4% of our children tested showed a low level of IgG in the blood compared to international standards.
A high level of IgG, especially in the early years of the child, can be provided by passive immunization of the mother and high endogenously production of clonal B cells that respond particularly to high exposure to microorganisms also due to the presence of many natural antibodies directed against disaccharide galactose, α-galactose (α-Gal), which is found as a terminal sugar on glycosylated cell surface proteins, and is produced in response to the production of this sugar by bacteria in the human intestine [13]. This IgG elevation may also be due to apparent viral hepatitis, autoimmune hepatitis and cirrhosis as described by Abbas et al. [14]. A higher percentage of current study children show a lower level of IgG in circulation (17.4%). This result can be explained by the high rate of factors that suppress the immune system in Yemen from malnutrition [15].
A secretory form of IgM has been found in body fluids and is transport through the epithelial lining is the same as that for secretory IgA [16]. In an immune response, IgM is the first antibody produced in both the primary and secondary responses. During fetal development, IgM is the first antibody class to be produced [17].
In the current study, most of the individuals tested (94.2%) showed the normal level of IgM in the circulation according to the international standard level (40-120mg/dl).There is no high level of immunoglobulin among the children in our study and this indicates that the children of the study are healthy, increases occur in IgM usually the following cases: Waldenstrom’s macroglobulinemia, trypanosomiasis, malaria, infectious mononucleosis, lupus erythematous, rheumatoid arthritis, dysgammaglobulinaemia (certain cases) [18]. While 8% of the children tested show a low level of immunoglobulin, this may be due to the prevalence of malnutrition among children in Yemen due to the Saudi-UAE aggression on Yemen.
Humoral responses induced in mucosal follicles result predominantly in the production of IgA antibodies, which have several unique properties that allow them to function efficiently in the mucosal environment [19]. In human the rate of IgA production is approximately twice that for IgG [20]. IgA is more abundant than other types of immunoglobulins, having a rate of excretion exceeding that of all other immunoglobulin combined when calculating its continuous secretion in humans.
In the current study, 57.6% of all children tested were at the normal level of IgA according to international standards (Table 4). However, 2.2% of children tested show a high level of serum IgA, and 40.2% of children currently tested show a low level of IgA in blood compared to international standards. IgA can mediate proinflammatory effects under certain circumstances. Thus, contact with IgA-coated particles can lead to opsonization and phagocyte activation through Fc receptors. Moreover, IgA interacts via its Fc region with lactoferrin and lactoperoxidase and thereby enhances the function of these innate host defense proteins [19]. Circulating IgA increases usually occur in the following conditions: Chronic, nonalcoholic liver diseases, especially primary biliary cirrhosis (PBC), obstructive jaundice, exercise, alcoholism, subacute and chronic infection. IgA decreases occur in the following conditions: Ataxia-telangiectasia, chronic sinopulmonary disease, congenital deficit, late pregnancy, prolonged exposure to benzene immunosuppressive therapy, and abstinence from alcohol after a period of 1 year, drugs and dextrin, protein-losing gastroenteropathies [21].
When IgG was calculated with age, it was found to increase in quantity with age at which the lowest concentration of IgG (637mg/dl) occurred in the age group of 0-12 months and the highest amount in the age group>13 years (1285mg/dl) (Table 5). The results of the present study are like those reported elsewhere where IgG serum concentration increases with age [22,23]. This result can be explained by the fact that IgG is provided at an early age by passive immunization of the mother and is produced by endogenous IgG produced by clonal B cells that respond specifically to antigens such as previous infection, vaccination, external exposure to other antigens, and increased quantity over time due to increased frequency of antigens exposure, due to any previous infection, vaccination or exposure of another foreign antigen [24]. Also, part of the amount of IgG is due to the production of natural antibodies by the immune system (mostly IgG) which are defined as antibodies produced without any previous infection, vaccination, exposure to other foreign antigens, or passive vaccination. These antibodies can activate the classical complement pathway leading to lysis of enveloped virus particles long before the adaptive immune response is activated. Many natural antibodies are directed against the disaccharide galactose α [25,26] galactose (α-Gal), which is found as a terminal sugar on glycosylated cell surface proteins and generated in response to production of this sugar by bacteria contained in the human gut [26].
Also, a large amount of IgG at a young age has a low affinity because of that at a young age, after activation with the antigen, B cells begin to multiply rapidly. In these rapidly dividing cells, genes that symbolize the variable domains in heavy and light chains pass at a high rate of point mutation, through a process called somatic hypermutation (SHM). SHM produces approximately one nucleotide in each variable gene, for each cell division [27]. As a result, any daughter cells will acquire slight differences in amino acids in the variable domains of their antibody chains. This increases the diversity of antibody assembly and affects the affinity of antibody binding to the antigen [28]. Some point mutations will produce antibodies with a weaker reaction (low affinity) with their antigen than the original antibody, and some mutations will generate stronger antibodies (high affinity) [29].
The crude mean of IgG levels in the children of the current study (Table 5) is close to the average in Oman [30] and Helsinki-Finland [31], but higher than that observed in Spain [18], Kashmir (India) [22], California [32], and less than Nigeria [33].
When IgM serum concentration considering with age, we found no significant difference related with age. (Table 6). Our result is like that reported elsewhere in which IgM serum concentration is not age related accept in 0-30 days infant in which geometric mean ± SD mg/dl of IgM is very low (18.5±3.5mg/dl) [34]. This result can be explained by that IgM in an immune response, IgM is the first antibody produced in both the primary and secondary responses and disappear rapidly after cure of infection [18]. When IgA serum concentration considering with age, a statistical analysis revealed a significant relationship between age and IgA concentrations up to ages 8 to 10 years (Table 7). Our result is like that reported elsewhere in which IgA serum concentration is age related and rise to the peak concentration in 7 and 8 years old [23]. Also, no correlation was present between age and immunoglobulin concentrations beyond that time, suggesting that the adult concentrations of IgA are normally reached and maintained after ages 7 and 8 years [23]. When concentration of immunoglobulins tested among our subjects, no truly significant differences were found in immunoglobulin concentrations (IgG, IgM, and IgA) which could be attributed to sex (Table 8). Our result is like that reported in Turkey and USA among children in whom they found no truly significant differences of immunoglobulin concentrations attributed to sex [35-37].
Conclusion
The study provided useful information on the normal level of serum immunoglobulins concentration in infants and children. The results can be considered as reference values for Yemen. Serum levels of IgG, IgM and IgA in most healthy children in our study were within the normal range of international standards, with an average value equal to that reported worldwide. Also, future studies should be conducted aimed at determining the values of IgG subcategories.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Van Kessel DA, Horikx PE, Van Houte AJ, De Graaff CS, Van Velzen-Blad H, et al. (1999) Clinical and immunological evaluation of patients with mild IgG1 deficiency. Clin Exp Immunol 118(1): 102-107.
- Aljedry ZAHS, Shaib AA, Al Shamahy HAH, Al Jaufy AY (2019) Tetanus immunization among pregnant women: coverage rate and rate of protection at time of delivery. Universal Journal of Pharmaceutical Research 4(1): 12-16.
- Al Kassar WYA, Alhadi Y, Al Shamahy HA, Al Hamzy M (2019) The Association of Elevated Serum IgE and Xerostomia with Recurrent Aphthous Stomatitis. On J Dent & Oral Health 2(1): 1-5.
- Nassar MY, Al-Shamahy HA, Al-Samawi AS, Abu Asba NW, El-Nono IH, et al. (2017) Human Leukocyte Antigen Class I and II Variants in Yemeni Patients with Chronic Renal Failure. Iran J Immunol 14(3): 240-249.
- Nassar MY, Al-Shamahy HA, AL-Barq AMM (2018) Evaluation of the immune response to polio vaccine in malnourished children in Sana’a city. Universal Journal of Pharmaceutical Research 3(2): 29-33.
- Nassar MY, Al-Shamahy HA, Haitham AA Masood (2015) The Association between Human Leukocyte Antigens and Hypertensive End-Stage Renal Failure among Yemeni patients. Sultan Qaboos Univ Med J 15(2): e241-e249.
- Aksu G, Genel F, Koturoğlu G, Kurugöl Z, Kütükçüler N (2005) Serum Immunoglobulin (IgG, IgM, IgA) and IgG subclass concentration in healthy children: A study using nephlometric technique. Turk J Pediatr 48(1): 19-24.
- Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. (2015) Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol 136(5):1186-205.
- Ballow M (2002) Primary immunodeficiency disorders: antibody deficiency. J Allergy Clin Immunol 109(4): 581-91.
- Stiehm ER, Ochs HD, Winkelstein JA (2004) Antibody Deficiencies. In: Ochs HD, Winkelstein JA, Stiehm ER (Eds), Immunologic Disorders in Infants & Children. Philadelphia: Saunders, pp. 357-369.
- Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, et al. (2009) Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol 124(6): 1161-1178.
- Notarangelo LD (2010) Primary immunodeficiencies. J Allergy Clin Immunol 125(2 Suppl 2): S182-94.
- Racaniello, Vincent (2009) Natural antibody protects against viral infection. Virology Blog.
- Abbas AK, Lichman AH (2003) Cellular and Molecular Immunology (5th edn), Saunders Chaina 3: 43-64.
- Abbas AK, Lichman AH (2004) Basic immunology (Functions and disorders of the immune system) (2nd edn) Saunder 4: 70-89.
- Dorthy JF (1999) Immunoglobulin Structure and function in Clinical Immunology (2nd edn), Philadelphia. New York 8: 91-102
- Talaro, Kathleen, Park, Talaro A (2001) Faundation in Microbiology; Publisher: Mcgraw-Hill College 4th
- Gonzalez-Quintela A, Alend R, Gude F, Campos J, Rey J et al. (2007) Serum levels of immunoglobulins ( IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol 151(1): 42-50.
- Stober Warren, Fuss Ivan J (2001) The mucosal Immune system. Medical immunology (10th edn), 14: 204-214.
- Yoo EM, Morrison SL (2005) IgA: An Immune glycoprotein. Clinical Immunology 116(1): 3-10.
- Goldsby RA, Kindt TJ, Osborne BA, Kuby J (2003) Immunology (5th edn) 4: 76-104
- Bhat GA, Mubarik M, Bhat YM (1995) Serum immunoglobulin profile in normal Kashmiri adults. J Postgrad Med 41(3): 66-69.
- Chapel H, Haeney M, Misbah S, Snowden N (2001) Essentials of clinical Immunology (4th edn), Blackwell Science Tokyo, Oxford, Maldef 1: 1-29
- Bouman A, Heinman MJ, Fass MM (2005) Sex hormones and the immune response. Human Reproduction 11: 411-423.
- Charles Janeway, Paul Travers, Mark Walport, Mark J Shlomchik (2001) Immunobiology. (5th edn) Garland Publishing, New York, USA.
- Pier GB, Lyczak JB, Wetzler LM (2004) Immunology, Infection, and Immunity 2004. ASM Press. ISBN 1-55581-246-5
- Diaz M, Casali P (2002) Somatic immunoglobulin hypermutation. Curr Opin Immunol 14(2): 235-240.
- Honjo T, Habu S (1985) Origin of immune diversity: genetic variation and selection. Annu Rev Biochem 54(1): 803-830.
- Or-Guil M, Wittenbrink N, Weiser AA, Schuchhardt J (2007) Recirculation of germinal center B cells: a multilevel selection strategy for antibody maturation. Immunol Rev 216: 130-141.
- White AG, Al- Riyami HA, Kuchipudi P, Darr AS (1997) Immunoglobulins, Immunoglobulin G Subclasses and complement in Adult Omanis. Annals of Saudi Medicine 17(1): 39-41.
- Aho K, Helioovaara M, Knekt P, Reunanen A, Palosuo T (1997) Serum immunoglobulins and the risk of rheumatoid arthritis, Ann Rheum Dis 56(6): 351-356.
- Stiehm ER, Fundenberg HH (1966) Serum levels of Immunoglobulins in Health and Disease: A survey. Pediatrics 37(5): 715-727.
- Akinosun OM, Arinola OG , Saimnu LS (2006) Immunoglobulin classes and liver function tests in Nigerian petrol attendants. Indian journal of occupational and environmental medicine 10(2): 58-61.
- Peng SL, Szabo SJ, Glimcher LH (2002) T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci 99(8): 5545-5550.
- Haroun M El-sayed MM (2007) Diagnostic value of immunoglobulin A and M antibodies as a disease marker for hepatitis A infection. Turkish Journal of Biochemistry 32(1): 22-27.
- Külhaş Çelik İ, Civelek E, Metin A, Giniş T, Toyran M, et al. (2019) Comparison of reference systems in the assessment of age-related serum. immunoglobulin levels in pediatric patients. Turk J Med Sci 49(1): 147-152.
- Sitcharungsi R, Bunupuradah T, Pornvoranunt A, Apornpong T, Ananworanich J, et al. (2013) Immunoglobulin values in healthy Thai children aged ≤ 24 months determined by nephelometry. Asian Pac J Allergy Immunol 31(4): 307-313.
-
Al Shamahy HA, Nassar MYH, Salah MM, AL Magrami RTF, Al Moyed KA. Immunoglobulin Levels (IgG, IgM And IgA): Normal Values for Healthy Infants and Children in Sana’a City-Yemen. Glob J of Ped & Neonatol Car. 1(5): 2019. GJPNC.MS.ID.000525.
Immunoglobulin, IgG, IgM, IgA, Normal Values, Infants, Children, Yemen, Immunoglobulin concentrations, Antibody deficiency
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






