 Review Article
Review Article
Insights into the Increased Dietary Levels of Brine Shrimp Artemia franciscana co fed with Microparticle Diets in the Rearing of Litopenaeus vannamei
Yathish Ramena1,2*, Vikas Kumar3, Ram Kurapati2, Grace Ramena2, Ayushma Sharma2, Femi J. Fawole3, Krishna P Singha3, Amit K Yadav3 and Thomas Bosteels1
1Great Salt Lake Brine Shrimp Cooperative, Inc., 1750 W 2450 S, Ogden, UT, 84401, USA
2Department of Aquaculture and Fisheries, University of Arkansas at Pine Bluff, 1200 University Dr, Pine Bluff, AR 71601.
3 Aquaculture Research Institute, University of Idaho, Moscow, ID, 83844, USA
Yathish Ramena, Great Salt Lake Brine Shrimp Cooperative, Inc., 1750 W 2450 S, Ogden, UT, 84401, USA Department of Aquaculture and Fisheries, University of Arkansas at Pine Bluff, 1200 University Dr, Pine Bluff, AR 71601, USA
Received Date: April 07, 2025; Published Date: April 15, 2025
Abstract
Despite the historical significance of Artemia franciscana as live food for many fish and shrimp larvae and efforts to co-feed live Artemia with micro-particulate feeds, the ideal co-feeding combination for early larval and post-larval stages of Litopenaeus vannamei remains unknown. In two independent larval experiments, six different concentrations of Artemia nauplii were investigated as a co-feed with two different commercial micro-particulate diets and fed to satiation with a range Artemia feeding levels were employed. In two consecutive experimental trials, postlarvae performance was assessed in terms of survival, growth, muscle gut ratio (MGR), osmotic stress test, fatty acid, amino acid, and whole-body composition. Overall, increased Artemia inclusion levels significantly improved survival during the hatchery cycle (PL15) up to a level of 5,900g of Artemia per million L. vannamei postlarvae produced. Additionally, survival further increased with increased inclusion of Artemia when continually co-feeding during extended nursery life stages up to PL 35. Biochemical analysis of body tissue and feeds were not able to offer an explanation for the improved survival rates recommending that additional research will be required to further elucidate the reasons for improved survival with increased levels of Artemia co-feeding. In summary, few studies have attempted to determine optimal levels of co-feeding of Artemia and particulate feeds for hatchery and nursery culture of L. vannamei. These findings strongly suggest that co-feeding with 4 kg to 6 Kg of Artemia per million fry produced is recommended for the successful culture of L. vannamei post larvae up to PL15 with commercially available micro-particulate feeds and suggest that even higher levels of Artemia co-feeding may be beneficial for later PL stages typically encountered in the nursery culture of L. vannamei. Additional research will be required to identify reasons for the increased survival obtained with these optimal Artemia co-feeding levels.
Keywords: Artemia cyst, Litopenaeus vannamei, postlarvae, growth, Mackay Marine MP diet, fatty acid, amino acid efficiency
Introduction
Globally, aquaculture production has increased almost 12-fold since 1980 and currently provides over half of the fish and shellfish directly consumed by humans [1] Shrimp farming is booming, with global aquaculture output growing faster than other agricultural sectors. A recent review by the National Fishery Institute during the Global Seafood Market Conference in Miami, Florida, USA (January 2018) estimates global shrimp production to reach 3.5 million metric tons in 2018. Pacific white shrimp (Litopenaeus vannamei) which is particularly suitable for intensive cultivation because of its rapid development, disease resistance and high growth rates, has accordingly been cultured in many countries [2,3] and is currently the most widely cultured species on the plannet [4]. Because shrimp is a highly valuable seafood commodity [5] marine shrimp farming is one of the most crucial commercial aquaculture activities in production value [6]. The most challenging aspect of shrimp larval rearing is the raising of larval and early post larval stages, because of the heavy reliance on live feeds due to their underdeveloped digestive systems [7]. Early stages of zoea respond to phytoplankton, with later stages responding to live feeding organisms such as rotifers and artemia. Artemia is widely accepted as live feed in commercial hatcheries due to its nutritional value, palatability, digestibility, versatility, perennial availability, salinity tolerance, dormant cysts availability, and ease of use. Generally, the nutritional composition includes protein (51-55%), fat (13-19%), carbohydrate (14-15%), and n-3 HUFA (3-15%) [8]. Additionally, unlike formulated feeds, live feeds have a high moisture content, making the meal more appealing, comestible, and digestible. They are also free swimming and thus better dispersed in the water column. The brine shrimp Artemia Nauplius is the primary live food organism utilized in commercial hatcheries for the production of penaeid shrimp post-larvae. Perhaps due to their tiny size and slow movement, predatory fish and shrimp larvae can easily feed on them, reflective of the natural predator-prey interaction. Improvements in feed production technology have allowed for the successful co-feeding of micro-particulate feeds with live feeds in the hatchery cycle of Litopenaeus vannamei. Although multiple authors have suggested a need to replace live feeds such as Artemia entirely with compound feeds, this has been hampered by inconsistency of supply and difficulty of use) [9] however, recent publications have demonstrated supply consistency as a result of sustainable harvest practices of Artemia resources such as Great Salt Lake [10,11] challenging the notion of supply inconsistency. Moreover, technological innovation has made the hatching and use of Artemia easier and more efficient. Additionally, other endeavors, such as the efforts by the International Artemia Aquaculture Consortium to further improve sustainable harvest practices and Artemia aquaculture are implemented to further improve supply consistency of Artemia [12]. Attempts to replace live feeds such as Artemia entirely with micro-particulate feeds have not resulted in improved or even equivalent survival and growth during the hatchery cycle of Litopenaeus vannamei. Anh et al.13 found that postlarvae of P. monodon fed a combination of commercial feed and artemia as a live feed supplement performed better than when fed either diet alone and concluded that feed containing dried or fresh biomass of Artemia can partially supplement live feeds for the hatchery larval rearing of P. monodon. Other studies have demonstrated the benefits of co-feeding and increased co-feeding of Artemia during the hatchery and nursery cycle of Litopenaeus vannamei and suggested increased ingestion, improved digestion, and enzyme modulation as a potential cause for the beneficial effects of Artemia feeding [13]. Sommer [14] studied the effect of co-feeding live feeds such as Artemia and Nematodes in the nursery culture of L. vannamei and found improved survival rates when co-feeding live feeds. Gamboa-Delgado and Le- Vay [15] was the only study we found that studies different levels of Artemia co-feeding in the hatchery cycle of P. vannamei. He found increased survival levels with increased co-feeding of Artemia when replacing up to 75% of carbon of the micro-particulate feeds by Artemia and then noticed a significant decrease in survival when feeding only Artemia. Zelaya et al [16] noted improvements in growth but not survival in the nursery culture of L. vannamei when providing Artemia as a supplement to micro-particulate feeds. Because there is a paucity of recent studies focused on determining optimal co-feeding regimes of live Artemia with commercially available micro-particulate feeds in the hatchery and nursery cycle of L. vannamei, this study was designed to evaluate the optimal co-feeding levels of Artemia nauplii for different life stages of L. vannamei post larvae. Additionally, body protein and amino acid composition were analyzed in an effort to elucidate potential causes of significant differences observed when using increased feeding levels of Artemia co-feeding.
Materials and methods
Feeding Trial 1:
Six different levels of Artemia nauplii as a co-feed with two different commercial micro-particulate diets, were used to rear Litopenaeus vannamei from Nauplii stage to post-larvae (PL) stages 8 to PL35. Post larvae performance was evaluated in terms of survival, growth, and whole-body composition at PL8 and PL15 (first trial), and at PL15 and PL35 (second trial) in two subsequent experimental trials. The Artemia nauplii (Instar I) regime for the first trial was designed with target feeding levels of Commercial diet 1000, and Mackay marine microparticle diet with 0, 1, 2, 4 and 8 Kg of dry Artemia per million PL produced (Kg/mm PL) using Artemia with a hatchability of 90%. These target Artemia feeding levels were calculated based on an anticipated post larval survival of 70%. After adjusting for actual survival rates, actual co-feeding levels of Artemia were 1.53, 0, 1.44, 2.57, 4.33 and 10.34 Kg/mm PL produced for above listed diets, respectively. Artemia cysts, at Instar I, were estimated based on the initial Litopenaeus vannamei nauplii stocking density and survival to be 1 Kg (Commercial-1000), 0 Kg (MP-0), 1 Kg (MP-1000), 2 Kg (MP-2000), 4 Kg (MP-4000), and 8 Kg (MP- 8000) per million L. vannamei larvae. The final cysts in gram were calculated for 416 L. vannamei nauplii as 0.02g, 0g, 0.02g, 0.03g, 0.06, and 0.12g per tank, respectively (Table 8).
Feeding Trial 2:
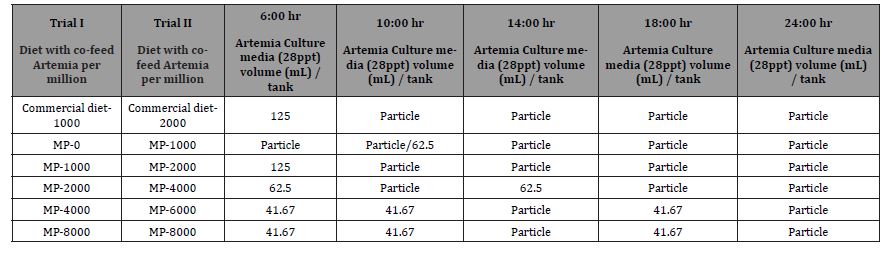
The second trial was conducted with a target feeding level of commercial diet with 2, 1, 2, 4, 6, and 8 Kg/mm PL through PL 15 and an additional target feeding level of 1.80, 0.90, 1.80, 3.60, 5.40, and 7.20 Kg/million PL from PL 15 through PL35. After adjusting using actual survival rates, the co-feeding levels of Artemia were at 2.83, 1.83, 2.64, 4.79, 5.95, and 8.05 Kg/mm PL produced through PL 15. And an additional 2.96, 1.88, 2.72, 5.02, 7.04, and 8.57 Kg/ mm PL produced from PL 16 through PL 35. Trials were run with the Mackay MP range of diets (MP) for all treatments. Additionally, another high end micro-particulate diet from a leading manufacturer of commercial hatchery feeds was used with one of the Artemia co-feeding levels to serve as a comparative reference to the Mackay line of MP diets. Whereas the Artemia feeding regime was fixed based on the target feeding levels, and micro-particulate diets were fed to satiation. Growth, survival, muscle to gut ratios, and amino acid and fatty acid profiles were evaluated for both trials. Artemia cyst at Instar I, were calculated for trial II (Table 9) as 0.04g, 0.02g, 0.04g, 0.08g, 0.12, and 0.16g per tank per day for Commercial-2000, MP-1000, MP-2000, MP-4000, MP-6000, and MP-8000, respectively. Newly hatched live Artemia nauplii were diluted and fed one to three times daily depending on the treatment to Larval Rearing Tanks (LRTs). Artemia feeding frequencies are given in Table 10. Clean suspensions of Artemia nauplii were delivered daily in LRTs. The number of algae and Artemia were recorded in LRT Data Log Sheets.
Experimental setup
At the beginning of the feeding trials, all the dietary treatments were color-coded and allocated to random tanks. Duplicate samples of commercial and MP diets and Artemia Instar I were collected aseptically for proximate, amino acid, and fatty acid analysis (Tables 6). Shrimp nauplii stage V were stocked into an indoor recirculating saltwater system comprised of 48 round plastic tanks (11.4-L each) at a stocking rate of 416 nauplii V per tank for trial I, and 650 nauplii per tank for trial II. Eight tanks were randomly assigned to each experimental diet, feeding is adjusted with the stocking density of the L. vannamei nauplii. During the first 2 days of the trial, shrimp were fed the experimental diets at 5.0% body weight (BW). Subsequently shrimp were fed the micro-particulate diets to satiation up to five times a day. Dechlorinated tap water was used to make artificial saltwater (30‰). All water quality parameters, including temperature, salinity, pH, dissolved oxygen, total ammonia nitrogen, and nitrite nitrogen were maintained within acceptable ranges for shrimp nauplii. Water temperature was maintained between 28-32°C with the use of an immersion heater, and continuous aeration was provided in individual tanks to maintain appropriate dissolved oxygen concentrations. A 12-h photoperiod was provided by artificial lighting controlled by timers. Tanks were cleaned daily. Mortality was recorded, and dead shrimp were removed. Feed intake was also recorded. Shrimp were sampled every 3 days to visually evaluate health, feeding behavior, fouling and necrosis during the study. At the end of the study, shrimp were sacrificed for the measurement of weight, survival, proximate analysis and whole-body amino acid and fatty acid composition.
Algae preparation. Thalassiosira psudonana, was diluted in 30 ppt saltwater at 28oC to 30,000 cells/mL from a stock solution of 565 million cells/mL and fed from Nauplii V to Zoea II stages. Subsequently, a mixture of Thalassiosira weissflogii at 15,000 cells/ mL and Thalassiosira. Psudonana at 15,000 cells/mL were provided from Zoea III to Mysis III stages.
Experimental diets
The proximate analysis, amino acid composition and lipid profile of the diets employed in these trials are listed in Table 6. A micro-particulate diet from a leading commercial brand (confidential), and Mackay Marine MP diet from Great Salt Lake Brine Shrimp Cooperative, Inc. were used in trials I and II.
Artemia
The Artemia cysts were obtained from the Great Salt Lake Brine Shrimp Cooperative, Inc. and hatched for 18h at the Aquaculture Research Institute, University of Idaho to determine percentage hatching capability per gram.
Water quality and culture condition
Water quality parameters, including chlorine content, pH, salinity, hardness, ammonia, nitrite, and nitrate parameters, were accessed for all tanks. Before stocking, excess ammonia levels were reduced to 0.1ppm using tetra-methyl amine. Subsequently, all tanks were supplemented with two doses of probiotic (500 billion CFU/g) at five ppm to stabilize the pH and ammonia. At stocking, salinity was recorded as 30 ppt. Each tank had individual water and air valves and was aerated by individual air stones supplied by a high-volume blower. All other culture conditions and system maintenance were as described in Gaylord and Rawles40. During the culture period, microalgae often clogged the 100-um mesh covering the top portion of the standpipe. A brush was used to clean the stand pipe screen, to avoid clogging. Tank temperature was maintained around 30 degrees. To ensure the culture system maintained acceptable temperature, dissolved oxygen (DO) and pH, these parameters were measured once daily in each tank at 0800–0900 am and 2 h postprandial using a hand-held multiprobe meter (Model HQ40D portable DO/pH meter, Hach Inc. Loveland, CO, USA). TAN was measured in water samples collected from each tank at 3 h postprandial using the Nessler Method on a portable spectrophotometer (Hach 49-1837-00 Water Hardness, and pH Test Kit HA62, Hach Inc). Pre-prandial tank DO average was 5.5 ± 0.03 mg/L, while the average postprandial DO was 6.48±0.21 mg/L with no distinct patterns with respect to treatment. Artemia were hatched for 18 hours in Imhoff cones at a water salinity of 28ppt and a temperature of 30oC. The treatment requirements for live, hatched Artemia nauplii were calculated for each trial (supplementary data, Table 2 and 3 Live Artemia nauplii samples were collected, inactivated, and preserved using 1ppm of 10% neutral buffered formalin solution for proximate analysis
Litopenaeus vannamei nauplii were received in two separate polyethylene bags with oxygen; nauplii were acclimatized during a period of 2 hours to adjust to the culture tanks’ temperature, salinity, pH, and mineral balance. Live Nauplii were counted using a dissection microscope with 5x magnification by counting three sample of from each bag prior to stocking.
Experimental facility and shrimp
The experiment was conducted in a closed recirculating aquaculture system (RAS) at the Aquaculture Research Institute, University of Idaho. L. vannamei nauplii stage 5 (N6) of Pacific white shrimp were obtained from Shrimp Improvement Systems, LLC (Islamorada, FL).
Sampling
At the end of the trial, shrimp from individual tanks were harvested and counted. Tank biomass was determined by measuring the weight of all animals after rinsing with tap water and removing any excess water with blotting paper. Fifty shrimp post larvae were sampled for determination of whole-body proximate analysis, amino acid and fatty acid analysis. Determination of muscle to gut ratio was performed several times during the culture period by randomly sampling ten shrimp from each tank. Individual wet weight was determined up to three times by randomly selecting twenty post larvae from each tank. Additionally, fifteen shrimp were sampled three times in order to determine survival under osmotic stress.
Calculations
Individual Wet weight: This was measured by the weight of post-larvae populations. Approximately 20 PLs samples were sampled, cleaned, rinsed with fresh water, and excess water was removed with blotting paper.
Tank weight gain (g) = mean final weight (Wf) - mean initial weight (Wi).
PL Muscle gut ratio (MGR): thickness of ventral abdominal muscle divided by the width of the gut at the sixth abdominal segment of the post larvae; a muscle to gut ration greater than 4 is indicative of a healthy nutritional status and development of the animal.
PL Necrosis: any necrosis in the body or signs of deformities were observed.
Stress tests: Osmotic stress: Mortalities were counted and reported as % mortality. The osmotic test was conducted three times during the experiment at PL-4, PL-7, and PL-12 stages.
Diets and whole-body proximate analysis, amino acids analysis and fatty acids analysis
Dry matter was determined after drying in a convection oven at 135°C for 2h (Isotemp 750F, Fisher Scientific, Hanover Park, Illinois, USA). Ash was determined by drying the sample at 600°C for 3 h [17] Crude Protein (N x 6.25) was determined by the Dumas method using a LECO nitrogen analyzer (FP428, LECO Corporation, St. Joseph, Michigan, USA). Crude fat in diets were determined by gravimetric quantification following hydrolysis in 3N HCL and petroleum ether extraction (AOCS 2009; Method AM 5-04) in an ANKOM × T15 lipid extractor (ANKOM Technology, Inc., Macedon, New York). Tissue lipids were similarly determined without the hydrolysis step. Diet and tissue samples were subjected to acid hydrolysis according to Rayne [18] without the addition of thioglycolic acid followed by amino acid analysis according to the methods of Henderson et al. [19] (2000) and Ramena et al. [20] (2020) on a high-performance liquid chromatography system (HP 1100; Agilent Technologies, Wilmington, Delaware, USA) equipped with a reversed- phase analytical column (Agilent Zorbax AAA 3.0 × 150 mm, 3.5 μm) and fluorescence detector (Hewlett-Packard G1321-A). The fatty acid composition of the diets and shrimp were determined using gas chromatography-mass spectroscopy following a modified AOAC [21] method 991.39 as described by Fawole et al [22].
Statistical Analysis of Data
Tank means were used as units of observation for statistical analysis. Shrimp growth data were analyzed for normality and homogeneity of variance prior to a one-way Analysis of Variance (ANOVA). If significant differences are found, data were subjected to the Duncan Multiple Range Tests test to separate the means at a significance level of p<0.05.
Results and Discussion
Trial 1:
Water quality parameters
During the trial period, the salinity of the culture ranged between 29 – 32 ppt in all the experimental tanks. The pH and temperature values were found to be in the range of 7.5 – 8.2 and 27 – 32 ºC, respectively, in all the tanks. Furthermore, the ammonia level in all the experimental tanks was found to range between 0 – 0.025 mg/L.
Survival, weight, length, muscle to gut ratio and osmotic stress test
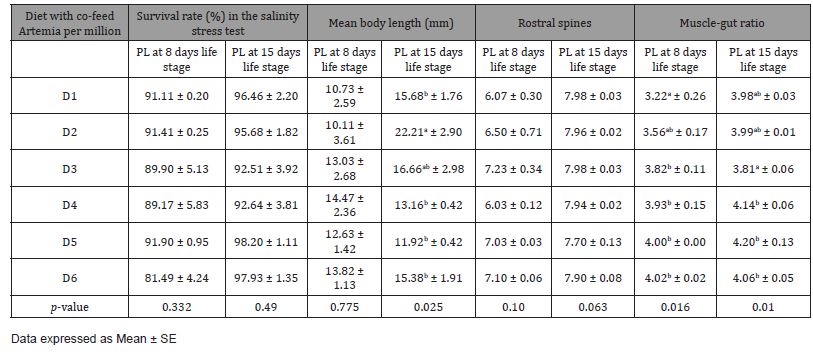
In the first trial, three culture tanks per group were harvested at PL8, and 5 culture tanks were harvested at PL15. At the end of the partial and final harvests, there was no significant difference (p>0.05) in the mean body weight (Figures 1 and 3) among the dietary treatments. There was however a significant difference (p<0.05) in the survival rate among the treatments (Figure 2). At both PL8 and PL15, all treatments in which Artemia was fed in conjunction with the MP diet, showed a significantly higher survival when compared to the treatment in which zero (0) Artemia was fed (Figure 2). There was no significant difference detected between the MP diet and the commercial diet, both fed a similar quantity of Artemia (D1 and D3). At PL 15, there was a clear differentiation between the different co-feeding levels of Artemia. Survival improved significantly (p<0.05) (Figure 4) as the artemia feeding level increased up to 4300g per million post larvae produced (50.4 Artemia nauplii/PL) (D5). At the highest inclusion rate of Artemia (10.34kg per million PL produced), survival decreased again similar to those fed D4 but better than MP diet-fed only group. Artemia inclusions did not affect the survival rates in the osmotic stress test that was conducted at PL-8 (p=0.332; Table 1) and PL15 (p=0.49; Table 1) life stages of Litopenaeus vannamei. Some significant differences (p<0.05; Table 1) were observed in muscle-gut ratios at both PL8 and PL15. In general, the muscle-gut ratio seemed to increase with increasing Artemia co-feeding although the differences were not always found to be significant. The mean body length at PL8 showed no differences among the groups (p>0.05, Table 1). At PL 15, a higher (p<0.05; Table 1) mean body length (22.21mm) was found in the dietary treatment provided with zero Artemia (D2) when compared to the feeding regimes in which Artemia was fed. In general, it appeared that the overall body length was inversely correlated with survival.
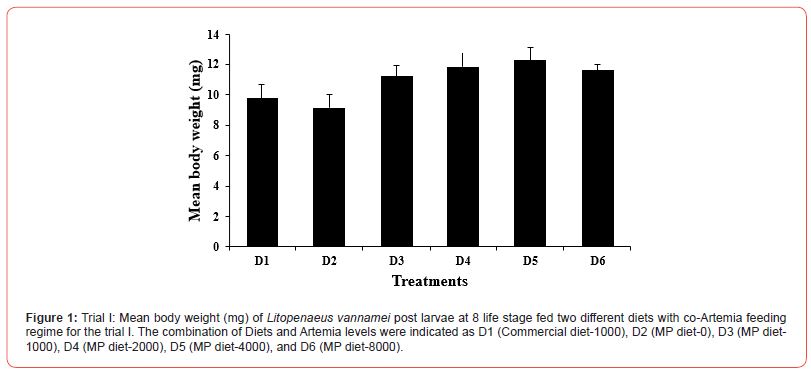
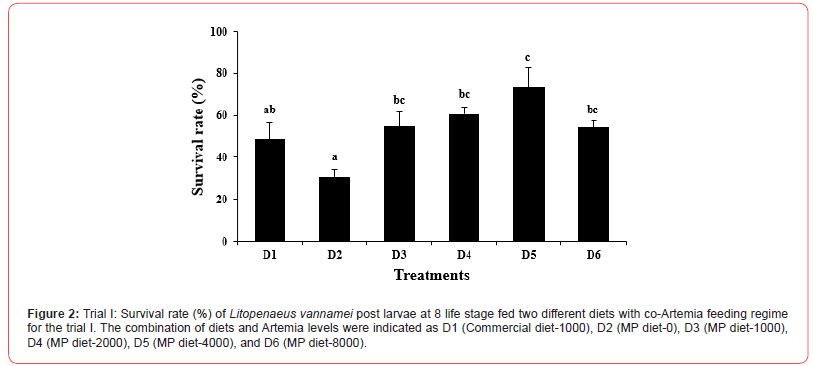
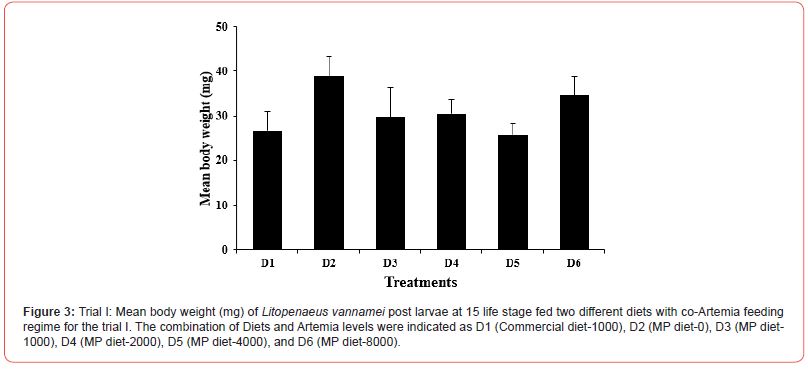
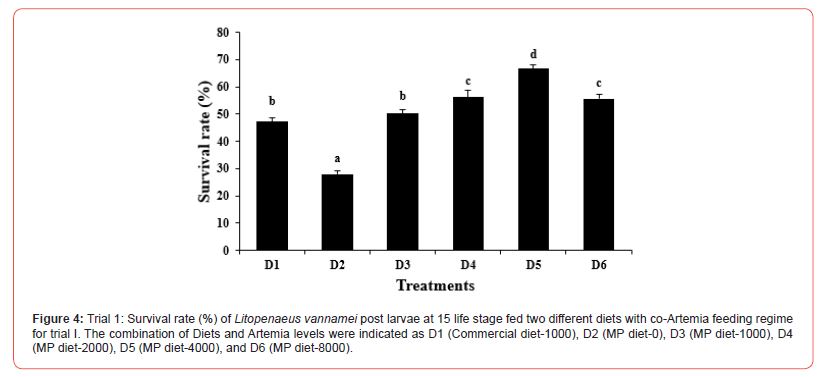
Table 1: Physical characteristics and osmotic stress test (%) of Litopenaeus vannamei post larvae at 8 and 15 life stage fed two different diets with co-Artemia feeding regime for the trial I. The combination of Diets and Artemia levels were indicated as D1 (Commercial diet-1000), D2 (MP diet-0), D3 (MP diet-1000), D4 (MP diet-2000), D5 (MP diet-4000), and D6 (MP diet-8000).
Whole-body protein, amino acid and fatty acid composition
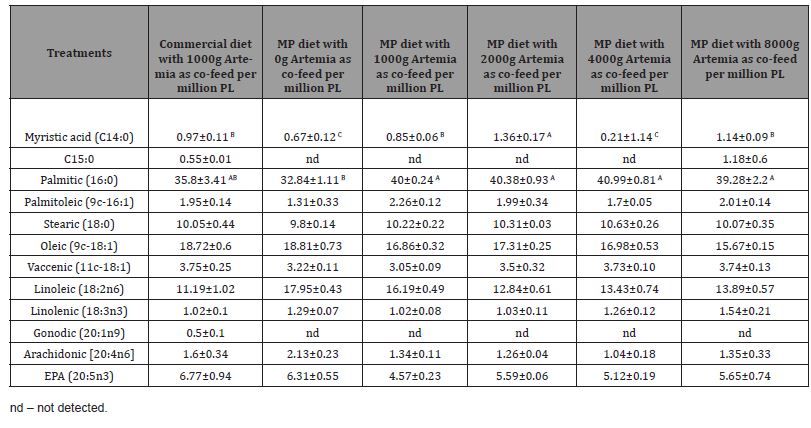
Whole-body crude protein (p=0.502), crude fat (p=0.4861), and amino acid composition were not affected by any dietary treatment (Table 2). Although we noticed a higher numerical value of crude protein and most limiting amino acids such as methionine, lysine, and threonine in the group fed the highest co-feeding levels of Artemia, these values were not found to be significant. Fatty acids in different dietary treatments of PL15 did not show any significant difference among the treatment groups except for myristic acid and palmitic acid (Table 3). The PL 15-fed MP diet with 2000g of artemia had the highest myristic acid deposition in the whole body compared to other dietary groups (p<0.05). Overall, there was no correlation between Artemia co-feeding levels and fatty acid profile of postlarvae reared up to 15 life stage (Table 3).
Table 2: Amino acid composition (as-is weight basis) of Litopenaeus vannamei post larvae 15 fed Commercial and MP diets with different Artemia co-feeding regime for the Trial I.
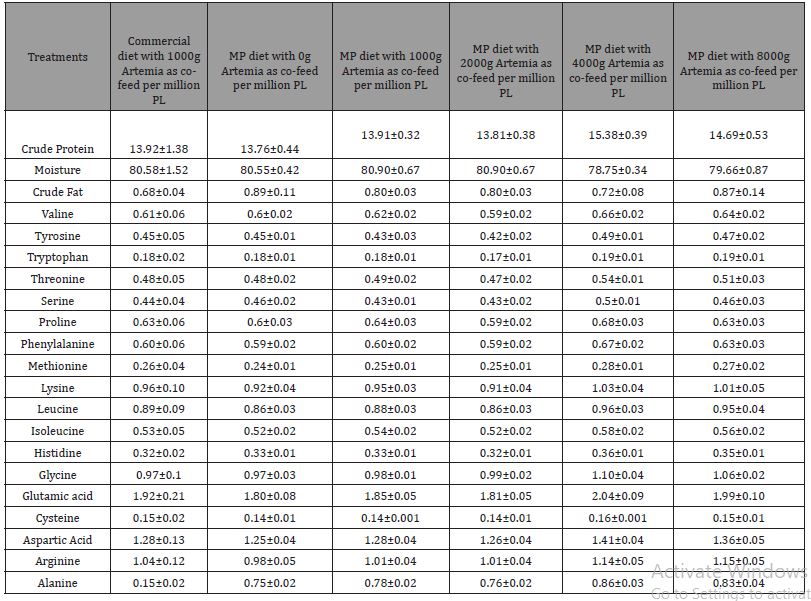
Table 3: Fatty acid composition (as-is weight basis) of Litopenaeus vannamei post larvae 15 fed Commercial and MP diets with different Artemia co-feeding regime for Trial I.
Trial II:
Survival, weight, length, muscle gut ratio (MGR), and osmotic stress test
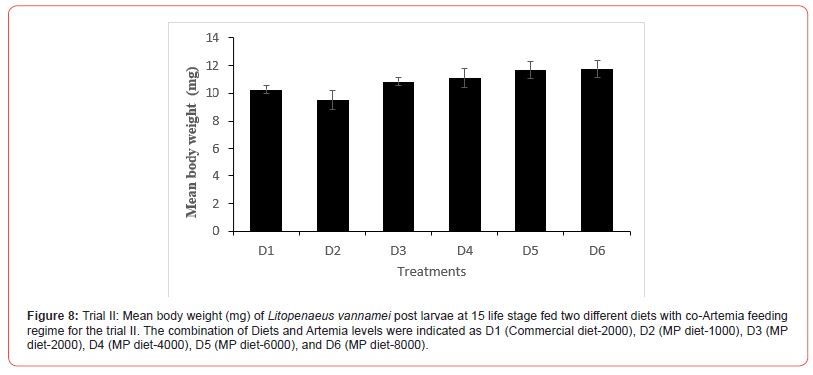
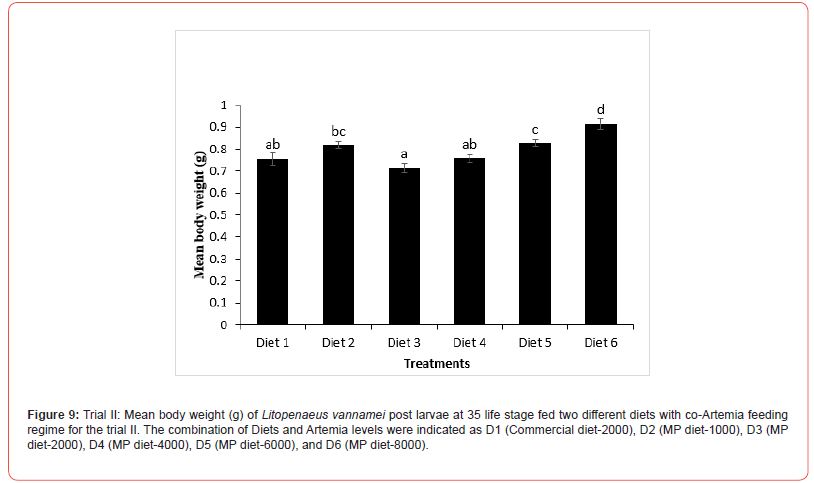
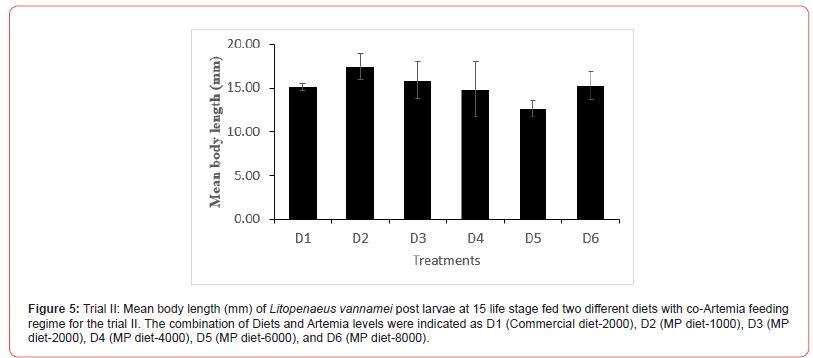
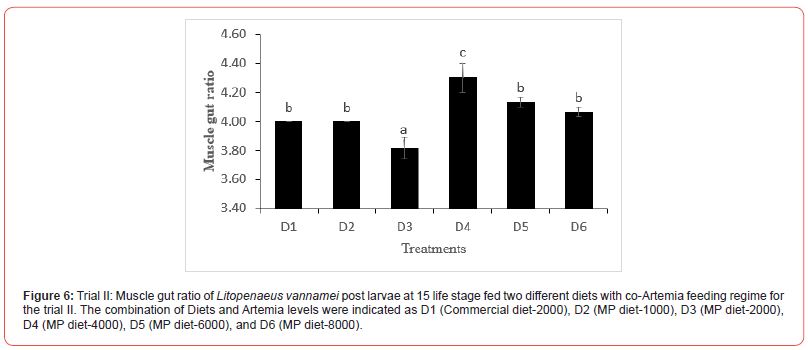
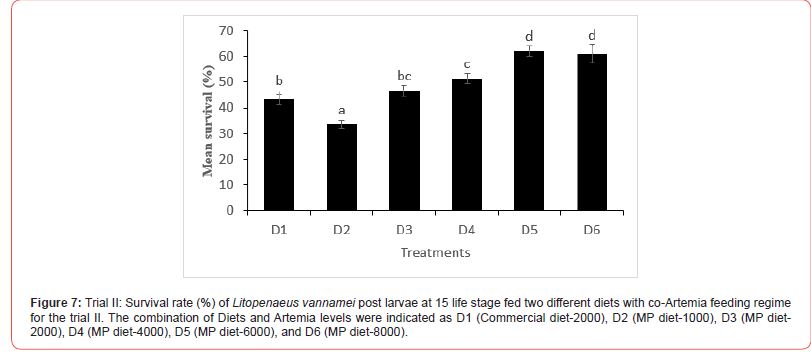
In this trial, three culture tanks were harvested at PL15. Culture was further extended to PL35 with the remaining five tanks for every treatment to evaluate the extended levels of Artemia co-feeding during the later PL stages on the growth indices of the post-larvae. The post larvae reared up to 15 did not show any significant mean body length (Figure 5), however, muscle-gut ratios improved significantly (4.2:1) for the tanks fed MP-4000 (Figure 6) but then decreased again at higher artemia feeding levels. Survival increased significantly at both life stages, PL 15 and PL 35, with increased co-feeding levels of Artemia (Figure. 5c and Figure 7). At PL 15 survival seemed to level off at the highest co-feeding level, suggesting that the optimal co-feeding of Artemia to support optimal survival at PL 15 is around 5,946 grams of Artemia per million PL produced (Figure 7). When continuing co-feeding of Artemia at higher PL stages (PL16 to PL35), optimal co-feeding of Artemia to support optimal survival continued to improve significantly up to the highest co-feeding level of 18.095 kg of Artemia per million PL produced (Figure 7). Overall, survival improved by about 84% when co-feeding Artemia at 5.9 kg/mm PL versus 1.8 kg/mmPL through PL 15. In contrast, survival for the combined hatchery and nursery cycle increased by about 75% when feeding up to 18,095 g/mmPL versus 4 kg/mmPL. An apparent, albeit not significant trend showing improvements in mean body weight with increased Artemia co-feeding, were noted at PL 15 (Figure 8). When culture was extended to PL35, mean body weight did result in significant differences at the highest Artemia co-feeding level as compared to all other dietary groups (Figure 9) ; p<0.05). Mean body lengths were not significantly different at PL 15 (p>0.05). At PL 35, there were some significant differences in mean body length, but these did not correlated with improvements in survival (Figure 10 and 11) or in mean body weight figure (Figure 9).
Whole-body protein and amino acid composition
Whole-body crude protein (p = 0.1319), moisture (p = 0.2117), and fiber (p = 0.4206) were not affected by the dietary treatments (Table 4); however, crude fat was significantly (p = 0.0123) lower in the group fed the MP diet co-fed with 6000g Artemia while other dietary groups had higher values, but no statistical differences were observed among the groups fed with commercial diet 2000, and MP diets with 1000g, 2000g, 4000g, and 8000g Artemia inclusion rates. Some of the essential and non-essential amino acids were found to be significant in the whole-body PL35 samples. For instance, valine, tyrosine, serine, leucine, isoleucine, and cysteine were significantly higher in the MP with 6000g Artemia fed group than in other treatments, while valine was lower in PL35 fed commercial diet 2000, MP-2000, and MP-4000 Artemia-based diet (Table 4). The wholebody essential and non-essential fatty acid profiles were affected significantly by the dietary Artemia levels (Table 5) when they were raised to the post-larvae 35 life stage. Commercial diet with 2000g of Artemia-fed PL had higher myristic acid (14:0) and palmitic acid (16:0), while linolenic (18:3n3) and EPA (20:5n3) were correlated with the high inclusion levels of Artemia treatments (Table 5). Arachidonic acid (20:4n6) and DHA (22:6n3) were not significantly different among dietary the dietary groups (Table 5). The tank water collected from each treatment was analyzed for elemental (macro and micro) analysis and was not affected by any dietary treatment, irrespective of the inclusion level (Table 7).
Table 4: Amino acid composition (as-is wet weight basis) of Litopenaeus vannamei post larvae 35 fed Commercial and MP diets with different Artemia co-feeding regime for the Trial II.

Table 5: Fatty acid composition (as-is wet weight basis) of Litopenaeus vannamei post larvae 35 fed Commercial and MP diets with different Artemia co-feeding regime for Trial II.
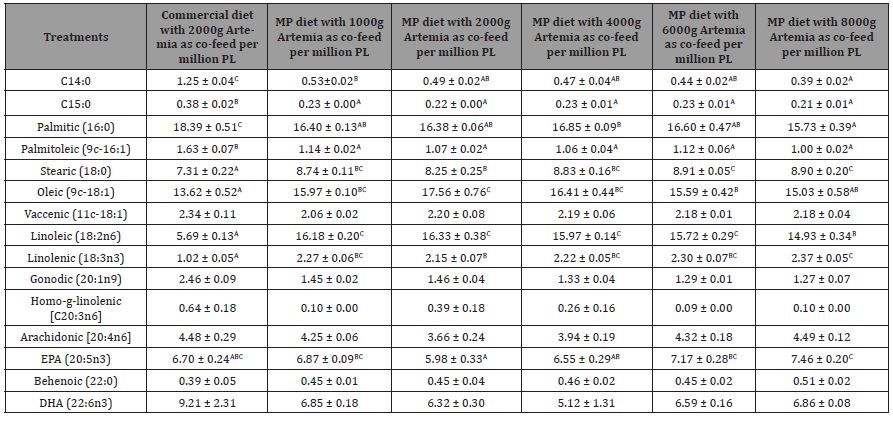
Table 6: Mean proximate composition (g/100g dry weight), amino acid, and fatty composition of MP, commercial diets and Artemia nauplii (Instar I stage). Values are means of duplicate samples.
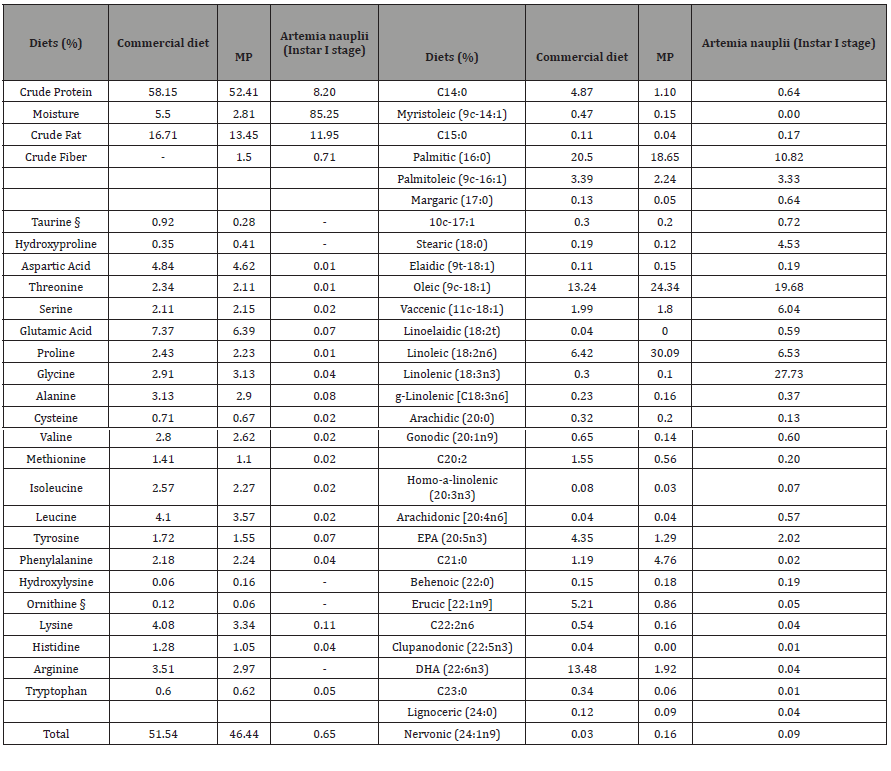
Table 7: Macro and micro elemental composition (ppb) (as-is wet weight basis) of culture water of Commercial diet, MP with different levels of Artemia co-feed to the Trial II dietary treatments
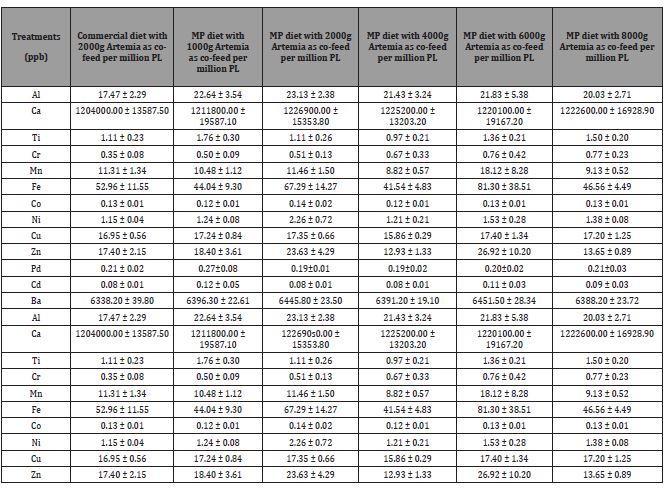
Table 8: Different Artemia feeding regime levels, estimated and actual cyst mass (g) for hatching per day for Trial I.
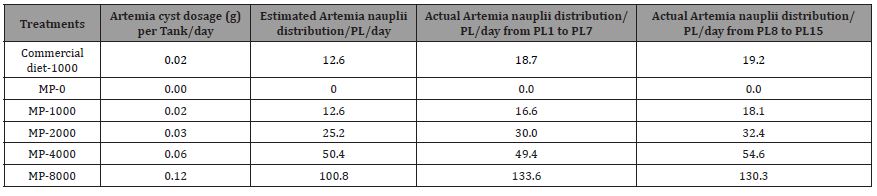
Table 9: Different Artemia co-feeding regime levels, estimated and actual cyst mass (g) for hatching per day for Trial II.
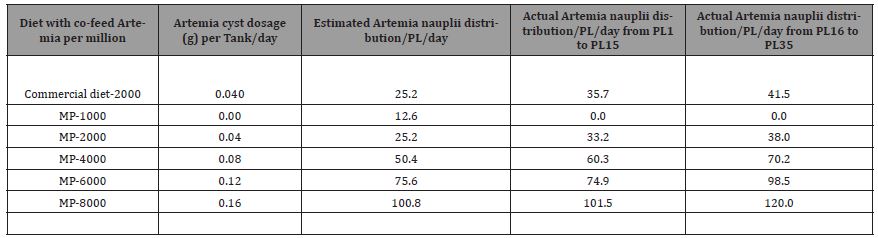
Table 10: Frequency of Artemia and particle using a culture media at 18h hatching for the trials I and II
Discussion
Shrimp farming is an important sector of the global aquaculture industry, and feeds play a vital role in the larval nutrition of shrimp. Micro feeding, either as a live feed or as a supplement in shrimp feed, has been shown to enhance growth performance and feed utilization, protein accretion for muscle growth, improved physiological condition and stress tolerance, better carcass quality, and survival23-25. Water quality variables play a significant role in aquatic animal productivity and are essential for the optimum growth of culture organisms, including L. vannamei [26]. Salinity is one of the most important water quality variables in shrimp hatcheries that influences several functional responses such as metabolism, reproduction, growth, osmoregulation, etc. In the current study, the salinity was between 29-32 ppt in all the experimental tanks, which is within the salinity range (28-35 ppt) recommended for shrimp hatchery [27-28]. Similarly, Krishnaprakash [29] and Karthik et al.30 also maintained the salinity for the larval rearing of Penaeus monodon and Litopenaeus vannamei at 31 and 30 ppt, respectively. During the culture period, the pH and temperature were found to range between 7.5-8.2 and 27-32 ºC in all tanks. This was in line with the reported acceptable range for penaeid culture [31-33] Furthermore, the ammonia concentration observed in all the experimental tanks was within acceptable range as reported by Khanjani et al. and Karthik et al. for P. monodon and L. vannamei larval culture. Poor water quality has been reported to cause growth reduction and an increased rate of mortality in shrimp [34- 36] and since the salinity, pH, temperature, and ammonia level of all the experimental tanks are within the optimum range, it could be inferred that the culture water for L. vannamei in the experimental set-up did not negatively impact the culture condition of the shrimp. Feeding of micro-particulate diet and Artemia nauplii along with algae was found to improve the growth performance, soluble protein content, and enhance the digestive enzyme response in L. vannamei postlarvae37. In the current study, no significant variation was observed in the body weight of L. vannamei at PL-15 irrespective of the diets fed; however, continuous feeding with Artemia co-feeding did show a significant improvement in the body weight at PL-35. The growth of L. vannamei at PL35 showed an increasing trend with increase co-feeding with from D3 (MP diet-2000) to D6 (MP diet-8000). This is similar to some other studies which have shown improvement in growth as a result of increased feeding of Artemia in combination with micro-particulate feeds38. The lower growth response recorded at a lower co-feeding level with Artemia compared with the commercial diet-2000 and MP diet-1000 could have resulted from cannibalistic behavior as suggested by Sommer14 and thus changed the nutritional composition of the total feed intake at low levels of Artemia co-feeding. In contrast with the growth results, survival in the first experiment increased with increasing levels of Artemia co-feeding except for the highest level of Artemia co-feeding (10.3 kg per mm PL produced) where survival decreased from 66.7 to 55.7%. In the second experiment, a similar survival effect was noticed through PL 15, with survival levelling off around 60% above a co-feeding level of 5.9 kg of Artemia per mm PL. These results strongly suggest that increasing levels of Artemia co-feeding improve survival in the hatchery cycle (up to PL15) until an optimal level of co-feeding is achieved between 4.3 and 5.9 kg of Artemia per mm PL15 produced, after which survival levels off and possibly even decreases at very high (10 kg/mm PL15) co-feeding levels of Artemia. When compared to feeding only microparticulate diets, survival was found to be more than doubled during the hatchery phase of L.vannamei, which clearly demonstrates the benefits of co-feeding Artemia with microparticulate feeds. Similar to our studies, Gamboa-Delgado and Le- Vay39 demonstrated a significant increase in survival during the early hatchery phase of L. vannamei (PL 5) when co-fed with Artemia as compared to a micro-particulate diet alone. When replacing more than 50 of the micro-particulate diet’s carbon by live Artemia nauplii, survival increased from 35% to well over 90% during the larval and early post larval stages (up to PL5). These findings as well as our study further suggest that the live feed Artemia contributes essential elements that support optimal survival of L. vannamei rearing up to PL 15.
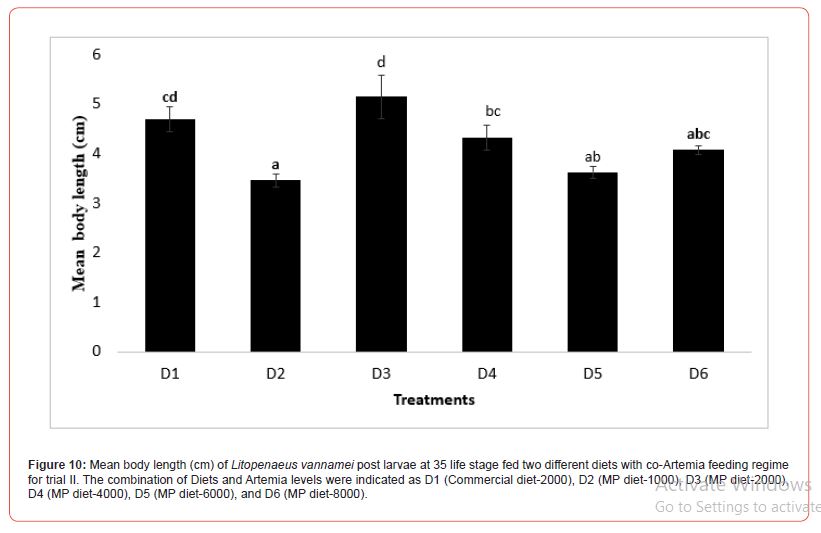
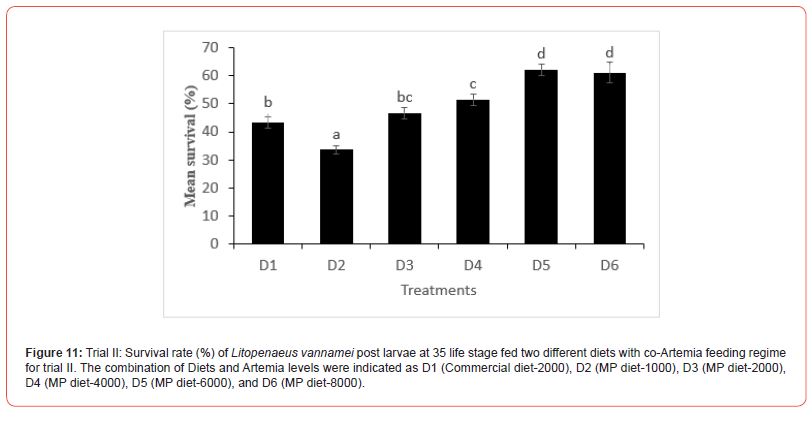
In the second trial, where the rearing was continued until PL 35, there appears to be further differentiation in survival between treatments. This is especially noteworthy in the case of the highest co-feeding level where survival of the two highest co-feeding levels of Artemia were not significantly different at PL 15, but later resulted in significant differences when co-feeding of Artemia was continued through PL 35. These results indicate that co-feeding of increasingly higher levels of Artemia could further enhance survival throughout the nursery phase of L. vannamei rearing (PL35). Our studies suggest that optimal feeding levels to enhance survival for the combined hatchery and nursery rearing cycle of L. vannamei are well in excess of 10 kg per mm PL 35 produced. Sommer14 did study the effects of live feeds during the nursery cycle of L. vannamei and showed significant improvements in survival when feeding live feeds such as Artemia and Nematodes in comparison to feeding inert feeds, however, the study did not attempt to research optimal levels of co-feeding of these live feeds. Additional studies will be necessary to isolate the nursery stage (PL 16 to PL 35) from the hatchery stage (up to PL15) in order to determine optimal Artemia co-feeding levels that could support enhance survival for individual production cycles.
In penaeids, a muscle gut ratio (MGR) of 4:1 has been reported to be an indication of healthy shrimp and good nutritional status40-41. In our study, although there appears to be an increasing trend of muscle to gut ratio with increased levels of Artemia co-feeding in trial I, these results were mostly not significant. Similarly, in trial II we observed some significant differences in muscle-gut ratios for the different treatment, but without a clear trend linking the MGR to the Artemia co-feeding level. These results, although suggestive that Artemia may play a role in optimizing the muscle-to-gut ratio in larval and post-larval rearing of L. vannamei, indicate that a further study is required to elucidate the possible importance of co-feeding Artemia for optimal development of L. vannamei muscle- to-gut ratio and associated shrimp health.
Whole-body composition analysis is a significant method for determining an animal’s nutritional condition, including shrimp. Claybrook42 noted that the concentration of free amino acids (FAA) in most crustaceans is higher than that in vertebrate tissues, which is possibly for osmoregulatory reasons42,43. In the present study, the whole-body crude fat levels considerably greater in the PL35 in the trial II when compared with the shrimp reared up to PL 15. The amino acid composition revealed that feeding higher levels of Artemia alongside MP increased whole-body deposition of cysteine. Furthermore, higher linolenic (18:3n-3), but similar levels of eicosapentaenoic acids (EPA; 20:5n-3) were recorded in MP co-fed with a similar inclusion level of Artemia compared to a commercial diet with 2000g of Artemia. In a related study, co-feeding of Artemia with Aurantiochytrium sp. significantly improved the body deposition of fatty acids such as gamma linolenic acid (C20:3n-6), C16:3n-3, linolenic acid (C18:3n-3), and stearidonic acid (C18:4n-3) in Penaeus monodon19. Similarly, enrichment of Artemia with Aurantiochytrium limacinum increased the total fatty acid content, primarily palmitic acid and DHA, in Penaeus vannamei42. The composition of dietary lipid fatty acids influences body lipid deposition in shrimp and fish [45-47] and long-chain polyunsaturated fatty acids (LC-PUFAs) have been shown to promote the growth and health of many larval marine animals25,44,46. In the present study, the whole-body fatty acid composition mirrored that of the diet, and the content of linolenic acid (18:3n-3), EPA, and DHA in the commercial diet was higher than that in the MP diet (Table 5). Also, the MP-based diet had more content of oleic (C18:1n–9) and linoleic (C18:2n–6) acids than the commercial diet. However, deposition of these fatty acids was observed in the whole body of PL 35 fed MP-based diets. This shows that the PL of L. vannamei adequately utilized the PUFA in the diet to biosynthesis LC-PUFA, producing a high level of EPA and DHA in the muscle even with a low dietary level of these PUFA’s, explaining how MP could be used for L. vannamei without compromising their LC-PUFA requirement even with lower dietary PUFA levels. This is similar to the work of Visudtiphole et al. [44], who after feeding Artemia with a thraustochytrid, observed higher EPA and DHA deposition in the muscle of P. vannamei. In addition, dietary replacement of fish oil with microalgae (Aurantiochytrium sp.) improved the muscle DHA level in L. vannamei [48].
Conclusion
In conclusion, the present study suggests an optimal co-feeding level of Artemia in combination with micro-particulate feeds that results in optimum survival of L. vannamei. It is evident from the current findings that co-feeding Artemia with a hatching percentage at or above 90% at levels between 4,332g- 5,946 g per million PL provides a diet that yields optimal survival levels through PL 15, typically representative of the hatchery rearing cycle of L. vannamei. Gross composition, amino acid analysis and fatty acid analysis were unable to explain the improved survival results with increased Artemia co-feeding. The comparison of the Mackay MP micro-particulate diet with another leading commercial diet did not result in significantly different survival or growth, further suggesting that the optimal co-feeding levels of Artemia may be applicable to other commercial micro-particle feeds presently available. Finally, the study also indicates that high levels of Artemia co-feeding well above 10kg per mm PL are also beneficial through the nursery culture of Artemia up to PL 35, although additional studies will be needed to determine optimal levels of Artemia co-feeding within the specific nursery culture of L. vannamei.
Acknowledgments
Great Salt Lake Brine Shrimp Cooperative, Inc. is an equal opportunity provider and employer. The mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply any extended recommendation by the GSLBSCI. We would like to extend our gratitude to Mr. Chad Vanderlinden for his early guidance.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This project was funded under a GSLBSCI extramural research agreement grant with the University of Idaho, Moscow, ID 83844.
References
- FAO (2017) FAO Statistics: Fishery and Aquaculture Statistics.
- Williams AS, Davis DA, Arnold CR (1996) Density‐dependent growth and survival of Penaeus setiferus and Penaeus vannamei in a semi‐closed recirculating system. Journal of the World Aquaculture Society 27(1): 107-112.
- Liu H, Wang L, Liu M, Wang B, Jiang K, et al. (2011) The intestinal microbial diversity in Chinese shrimp Fenneropenaeus chinensis as determined by PCR–DGGE and clone library analyses. Aquaculture 317(1–4): 32–36.
- Treece GD, Davis DA (2000). Culture of small zooplankters for the feeding of larval fish. SRAC Publication 701: 1–8.
- Anand PS, Kohli MPS, Roy SD, Sundaray JK, Kumar S, et al. (2013) Effect of dietary supplementation of periphyton on growth performance and digestive enzyme activities in Penaeus monodon. Aquaculture 392: 59-68.
- FAO (2010) The state of world fisheries and aquaculture: Opportunities and challenges. Food and Agriculture Organization of the United Nations, Rome.
- Agh N, Sorgeloos P (2005) Handbook of protocols and guidelines for culture and enrichment of live food for use in larviculture. Ediciones Artemia and Aquatic Animals Research Center, Urmia-Iran.
- Treece GD, Davis DA (2000) Culture of small zooplankters for the feeding of larval fish. SRAC Publication 701: 1-8.
- Madkour K, Dawood M, Sewilam H (2022) The use of Artemia for aquaculture industry: An updated overview. Annals of Animal Science 23(1).
- Marden B, Brown P, Bosteels T (2020) Great Salt Lake Artemia: ecosystem functions and services with a global reach. Great Salt Lake Biology: A Terminal Lake in a Time of Change 175-237.
- Belovsky GE, Perschon WC (2019) A management case study for a new commercial fishery: brine shrimp harvesting in Great Salt Lake, Utah, USA. Ecological Applications 29(3).
- IAAC (2019) Conservation, management and sustainable utilization of Artemia biodiversity.
- Anh NTN, Wille M, Van Hoa N, Sorgeloos P (2011) Formulated feeds containing fresh or dried Artemia as food supplement for larval rearing of black tiger shrimp (Penaeus monodon). Journal of Applied Aquaculture 23(3): 256–270.
- Sommer NP (2019) Evaluation of nematodes and artificial Artemia as feed for Pacific white shrimp in a biofloc nursery system (Doctoral dissertation). Universidade do Algarve (Portugal).
- Gamboa-Delgado J, Le Vay L (2009) Artemia replacement in co-feeding regimes for mysis and postlarval stages of Litopenaeus vannamei: Nutritional contribution of inert diets to tissue growth as indicated by natural carbon stable isotopes. Aquaculture 297(1-4):128-135.
- Zelaya O, Davis DA, Rouse DB (2007) The influence of Artemia and algal supplements during the nursery phase of rearing Pacific white shrimp Litopenaeus vannamei. Journal of the World Aquaculture Society 38(4): 486-496.
- AOAC 1990 Official Methods of Analysis, 15th ed. Association of Official Analytical Chemists, Arlington, VA pp. 1298.
- Rayner CJ (1985) Protein hydrolysis of animal feeds for amino acid content. Journal of Agricultural and Food Chemistry 33(4): 722–725.
- Henderson JW, Robert DR, Brian AB, Cliff W (2000) Rapid, Accurate, Sensitive and Reproducible HPLC Analysis of Amino Acids. Agilent Technologies, Application Note, Publication No: 5980-1193.
- Yathish Ramena, Steven D Rawles, Rebecca Lochmann, Gibson Gaylord T, Matthew E McEntire, et al. (2020) Growth, nutrient retention, innate immune response, and intestinal morphology of juvenile, soy-naïve hybrid striped bass, Morone saxatilisx chrysops fed commercial-type, soy-based, ideal protein, fish meal replacement diets, Aquaculture Volume 522: 735150.
- AOAC (1995) Official methods of analysis. 16th ed. Washington, DC: AOAC; 1995.
- Fawole FJ, Labh SN, Hossain MS, Overturf K, Small BC, et al. (2021) Insect black soldier fly larvae oil as a potential substitute for fish or soy oil in the fish meal-based diet of juvenile rainbow trout Oncorhynchus mykiss. Animal Nutrition 7(4): 1360–1370.
- Vizcaíno AJ, López G, Sáez MI, Jiménez JA, Barros A, et al. (2014) Effects of the microalga Scenedesmus almeriensisas fishmeal alternative in diets for gilthead sea bream Sparus aurata Aquaculture 431: 34–43.
- Rehberg-Haas S, Meyer S, Tielmann M, Lippemeier S, Vadstein O, et al. (2015) Use of the microalga Pavlova viridisas enrichment product for the feeding of Atlantic cod larvae Gadus morhua. Aquaculture 438: 141-150.
- Jaseera KV, Ebeneezar S, Sayooj P, Nair AV, Kaladharan P (2021) Dietary supplementation of microalgae, Aurantiochytrium and co-feeding with Artemiaenhances the growth, stress tolerance and survival in Penaeus monodon Fabricius, 1798 postlarvae. Aquaculture 533: 736176.
- Karthik R, Pushpam AC, Ramalingam K, Yuvaraj D, Vanitha M (2015) Attenuation of negative impacts by micro algae and enriched Artemia salinaon Penaeus monodon and Litopenaeus vannamei larval culture. Journal of Fisheries and Aquatic Science 10(5): 347.
- Van Wyk P, Scarpa J (1999) Water quality requirements and management. Farming Marine Shrimp in Recirculating Freshwater Systems 13: 128–138.
- Kannupandi T, Soundarapandian P, Anantharaman P (2002) Hatchery operation manual for Penaeus monodon Fabricius. Annamalai University. CAS in Marine Biology Parangipettai 1-99.
- Krishnaprakash R (2007) Studies on the production of high-quality shrimp Penaeus monodonFabricius seed in a commercial hatchery (Doctoral dissertation, M. Phil thesis, CAS in Marine Biology, Annamalai University, India, 1: 45.
- Karthik R, Pushpam AC, Ramalingam K, Yuvaraj D, Vanitha M (2015) Attenuation of negative impacts by micro algae and enriched Artemia salinaon Penaeus monodon and Litopenaeus vannamei larval culture. Journal of Fisheries and Aquatic Science 10(5): 347.
- Cohen JM, Samocha TM, Fox JM, Gandy RL, Lawrence AL (2005) Characterization of water quality factors during intensive raceway production of juvenile Litopenaeus vannameiusing limited discharge and biosecure management tools. Aquacultural Engineering 32(3-4): 425-442.
- Khanjani M, Sajjadi M, Alizadeh M, Sourinejad I (2016) Study on nursery growth performance of Pacific white shrimp Litopenaeus vannameiBoone, 1931 under different feeding levels in zero water exchange system. Iranian Journal of Fisheries Sciences 15(4): 1465-1484.
- Reddy MH, Mounika K (2018) Determination and comparative study of water quality parameters in shrimp culture ponds. International Journal for Research in Applied Science and Engineering Technology 6(9): 216-221.
- Páez-Osuna F, Gracia A, Flores-Verdugo F, Lyle-Fritch LP, Alonso-Rodrıguez, et al. (2003) Shrimp aquaculture development and the environment in the Gulf of California ecoregion. Marine Pollution Bulletin 46(7): 806-815.
- Anand PS, Balasubramanian CP, Christina L, Kumar S, Biswas G, et al. (2019) Substrate based black tiger shrimp, Penaeus monodonculture: Stocking density, aeration and their effect on growth performance, water quality and periphyton development. Aquaculture 507: 411–418.
- Eddiwan E, Sukendi S, Siregar YI, Saam Z (2020) The Effect of Water Quality Variables on Vannamei Shrimp Productivity Litopenaeus Vannamei in the Mining Area of the Sungai Pinang Village, Lingga Timur District, Lingga Regency, Riau Islands Province. IOP Conference Series: Earth and Environmental Science 430(1): 012039.
- Brito R, Rosas C, Chimal ME, Gaxiola G (2001) Effect of different diets on growth and digestive enzyme activity in Litopenaeus vannameiBoone, 1931 early post‐larvae. Aquaculture Research 32(4): 257–266.
- Gamboa-Delgado J, Le Vay L (2009) Artemia replacement in co-feeding regimes for mysis and postlarval stages of Litopenaeus vannamei: Nutritional contribution of inert diets to tissue growth as indicated by natural carbon stable isotopes. Aquaculture 297(1-4), 128–135.
- Le Vay L, Jones DA, Puello-Cruz AC, Sangha RS, Ngamphongsai C (2001) Digestion in relation to feeding strategies exhibited by crustacean larvae. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 128(3), 621–628.
- Saurabh S, Kumar V, Karanth S, Venkateshwarlu G (2006) Selection of high-health postlarvae: A prerequisite for sustainability of the Indian shrimp industry. Aquaculture Asia 11(2): 4.
- Uddin SA, Kader MA, Sikder M, Hakim MA, Alam MM, et al. (2013) Study of probiotics on the seed production of black tiger shrimp Penaeus monodon. Croatian Journal of Fisheries: Ribarstvo 71(3): 124–130.
- Claybrook DL (1983) Nitrogen metabolism. In: Mantel, L.H. (Ed.), The Biology of Crustacea, vol. 5, Internal Anatomy and Physiological Regulation. Academic Press, New York, pp. 163-213.
- Awapara, J (1962) Free amino acids in invertebratres: A comparative study of their distribution. Amsterdam: Elsevier ed. J. T. Holden, pp. 158-175.
- Visudtiphole V, Phromson M, Tala S, Bunphimpapha P, Raweeratanapong T, et al. (2018) Aurantiochytrium limacinumBCC52274 improves growth, hypo-salinity tolerance and swimming strength of Penaeus vannamei Aquaculture 495: 849–857.
- González‐Félix ML, Perez‐Velazquez M, Quintero‐Alvarez JM, Davis DA (2009) Effect of various dietary levels of docosahexaenoic and arachidonic acids and different n‐3/n‐6 ratios on biological performance of Pacific white shrimp, Litopenaeus vannamei, raised in low salinity. Journal of the World Aquaculture Society 40(2): 194–206.
- Soller F, Roy LA, Davis DA (2019) Replacement of fish oil in plant‐based diets for Pacific white shrimp, Litopenaeus vannamei, by stearine fish oil and palm oil. Journal of the World Aquaculture Society 50(1): 186–203.
- Fawole FJ, Labh SN, Hossain MS, Overturf K, Small BC, et al. (2021) Insect black soldier fly larvae oil as a potential substitute for fish or soy oil in the fish meal-based diet of juvenile rainbow trout Oncorhynchus mykiss. Animal Nutrition 7(4): 1360-1370.
- Guimarães AM, Dias Schleder D, Nagata M, Nóbrega RO, Fracalossi DM, et al. (2019) Aurantiochytrium meal can replace fish oil in practical diets for the juvenile Pacific white shrimp. Aquaculture Nutrition 25(4): 798-807.
-
Yathish Ramena1*, Vikas Kumar, Ram Kurapati, Grace Ramena, Ayushma Sharma, Femi J. Fawole, Krishna P Singha, Amit K Yadav and Thomas Bosteels. Insights into the Increased Dietary Levels of Brine Shrimp Artemia franciscana co fed with Microparticle Diets in the Rearing of Litopenaeus vannamei. 5(4): 2025. GJNFS.MS.ID.000618.
-
Mentha suaveolens Erh; cell stress response; melanogenesis inhibition; skin lightening
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






