 Research Article
Research Article
Contamination Status of Polycyclic Aromatic Hydrocarbons (PAH s) In Atmospheric Particulate Matter PM2.5 Samples of a Semi-Residential Area of Dhaka, Bangladesh
YN Jolly1*, A Tabassum2, MA Rahman2, MS Rahman 1, Sohel Rana3, S Akter1 and BA Begum1
1Atmospheric and Environmental Laboratory, Chemistry Division, Atomic Energy Centre, Dhaka
2Departmentof Chemistry, Dhaka University, Bangladesh
3Organic Chemistry Laboratory, Chemistry Division, Atomic Energy Centre, Dhaka
YN Jolly, Atmospheric and Environmental Laboratory, Chemistry Division, Atomic Energy Centre, Dhaka, Bangladesh.
Received Date: August 04, 2020; Published Date: November 11, 2020
Abstract
This study deals with the determination of the polycyclic aromatic hydrocarbons (PAHs) in the atmospheric particulate matters (PM2.5) at a semi residential site of Gazipur, Dhaka, Bangladesh. Source identification and possible human health impact of polycyclic aromatic hydrocarbon was evaluated as well. A total of 20 samples were collected in six weeks period of time. Polycyclic Aromatic Hydrocarbons (PAH’s) were determined using gas chromatography-mass spectrometry. The average concentration of Anthracene, Phenanthrene, Pyrene, Chrysene, Benzo (a) anthracene, Benzo(a)pyrene, perylene were found to be 0.309, 0.159, 0.227, 2.120, 1.954, 2.269 and 3.373μgm-3 respectively. Two-way ANOVA test revealed that the concentration of different PAHs species (Fcal> Fcrit) are significantly different from each other at a 95% confidence level. The main contributory sources for PAHs were found gasoline exhaust, diesel exhaust, wood burning and brick kilns. The result revealed that these compounds are present in a higher level in the atmosphere when compared with the value of other countries in the world. Concentration of highly carcinogenic Benzo(a) pyrene was in a range where carcinogenic effect is an immediate threat in case of long-time exposure and hence regular monitoring is suggested.
Keyword: Polycyclic Aromatic Hydrocarbon; Air-Metrics Mini-Vol Samplers; Gas Chromatography-Mass Spectrometry; Quartz Filters
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are organic compounds consist of two or more fused benzene rings in a linear or cluster arrangement, typically found as a complex mixtures [1]. They are very stable organic pollutants that are made up of only carbon and hydrogen and occur naturally but, they can be synthesized as individual compounds for research purposes. Furthermore, they have high boiling and melting points with high molec ular weights and are able to survive at high temperatures from the combustion of fuel from automobiles and airplanes engines and most of them have low water solubility [2]. Polycyclic aromatic hydrocarbons (PAHs) are considered ubiquitous in the environment and can be formed from either natural or manmade combustion sources [3]. The dominant sources of PAHs in the environment are thus from human activity: wood-burning and combustion of other bio-fuels etc., and wildfires are another notable source. Dungor crop residues contribute more than half of annual global PAHs emissions, particularly due to bio fuel use in India and China (Anita and Maharaj,2004), industrial processes and the extraction and use of fossil fuels made up slightly more than one quarter of global PAHs emissions, dominating outputs in industrial countries such as the United States. Lower-temperature combustion, such as tobacco smoking tends to generate low molecular weight PAHs, whereas high-temperature industrial processes typically generate PAHs with higher molecular weights [4].
Atmospheric PAHs are distributed (Figure 1) between the gas and particulate phases depending on their physicochemical properties. They can be transported through the atmosphere over long distances. Polycyclic Aromatic hydrocarbons are emitted into the atmosphere either as vapors or associated with primary aerosol particles. Once enters in the atmosphere, the residence times and ultimate fates of these semi volatile chemicals depend upon their distributions among vapor, particle, and droplet phases. The atmospheric chemical and photochemical reactions of PAHs are of great importance because the decomposition product of the PAHs may be more hazardous to human health than the PAHs from which they were derived [5]. A number of experimental studies have demonstrated that many PAHs are susceptible to photochemical and/or chemical oxidation under simulated atmospheric conditions [6]. Nitro PAHs are emitted as a result of incomplete combustion processes.
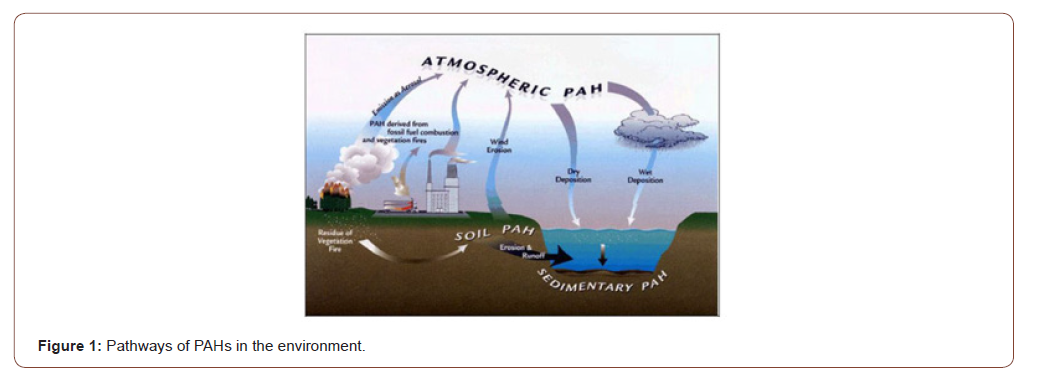
PAHs have been linked to different cancers in well-established animal model studies [7]. The structure of a PAH influences whether and how the individual compound is carcinogenic [8]. Some carcinogenic PAHs are genotoxic and induce mutations that initiate cancer; others are not genotoxic and instead affect cancer promotion or progression [9] and hence continued research regarding the mutagenic and carcinogenic effects from chronic exposure to PAHs and metabolites is needed. Other than carcinogenic, adult exposure to PAHs has been linked to cardiovascular disease as well [10]. PAHs are among the complex suite of contaminants in tobacco smoke and particulate air pollution and may contribute to cardiovascular disease resulting from such exposures [11]. laboratory experiments, animals exposed to certain PAHs have shown increased development of plaques (atherogenesis) within arteries [12]. Oxidative stress following PAH exposure could also result in cardiovascular disease by causing inflammation, which has been recognized as an important factor in the development of atherosclerosis and cardiovascular disease [13,14]. Biomarkers of exposure to PAHs in humans have been associated with inflammatory biomarkers that are recognized as important predictors of cardiovascular disease, suggesting that oxidative stress resulting from exposure to PAHs may be a mechanism of cardiovascular disease in humans [15]. Multiple epidemiological studies of people living in Europe, the United States, and China have linked in uterus exposure to PAHs, through air pollution or parental occupational exposure, with poor fetal growth, reduced immune function, and poorer neurological development, including lower IQ.
As PAHs are known to have carcinogenic, mutagenic and teratogenic properties, their persistence in the environment have been placed them on the list of priority pollutants by the United States Environmental Protection Agency (US-EPA) and also the European Environment Agency [16]. People from all over the world are concerned more about the air pollution aspects due to the increased rate of mortality and morbidity and also multifarious effects of particulate pollution and we are not out of it. In this regard it is imperative to have a systematic study ascertaining the facts concerning the nature, sources, and trends of the particulate pollution in our beloved city, Dhaka, Bangladesh.
Gazipur area of Dhaka, Bangladesh is known to have moderately dense in population with high traffic and other industrial establishments like garments factories etc. There are several brick kilns in and around the area; more over there is a very busy rail station. Different types of industrial and regular activities are there responsible to contribute a lot carcinogenic aromatic polycyclic hydro carbons in the air of that area that ultimately affects local habitants as well as the visitors. Present study therefore sketched to determine the polycyclic aromatic hydrocarbon concentration in the atmospheric particulate matters collected from Gazipur, Dhaka, Bangladesh, identification of possible sources and human health impact. The main objectives are therefore:
• Determination of the polycyclic aromatic hydro-carbons concentration in the atmospheric particulate matters of Gazipur air
• Identification of the source of the polycyclic aromatic hydrocarbon in the atmosphere.
• Understanding the possible human health impact of polycyclic aromatic hydrocarbon.
Materials and Methods
Sampling site
Air samples were collected from Gazipur area of Dhaka, Bangladesh, which is a residential area of moderate population density. The sampling location is within 20 m from a local road and about 200m from a secondary roadwith moderate traffic density. The highway of Joydeb puris a very busy traffic point, which is about 5 km west to the studied site. Joydebpur rail station, through which daily 60 trains pass away, is about 100m away from the sampling location. At “Konabari” and “Kodda” which are about 5 to 7 km to the north-west of the sampling site, more than 100 brick kilns are there in production using kindle wood. There are also many garments and other industrial units at 4 to 7 km distance from this site (Figure 2).
Samples collection and preparation
PM2.5 (particulate matter) sampling was started from 13 January 2014 by Air-Metrics Mini-Vol samplers at Joydebpur (Gazipur), Dhaka, Bangladesh. And the samplers were placed on the flat roof of the continuous air monitoring station (CAMS-4) site of Clean Air and Sustainable Environment (CASE) project, Gazipur city corporation central symmetry, at 20 feet height from the ground level. The amount of air passed was maintained at 7.2m3.PM2.5 was collected simultaneously for every 24 hours (from 10 a.m. to 10 a.m. of the next day) at the sampling site. The pre-weighted conditioned clean filters (quartz) were loaded to respective filter holder assembly at the conditioning room of CAMS. After sampling, filter holder assemblies (keeping the exposed filters inside) were brought to the conditioning room of the Atomic Energy Centre (AEC), Dhaka, directly from the sampling site for conditioning and PM filter retrieval. Special care was taken in transporting the exposed filter holder assemblies, so that there should be no PM loss.PM2.5 masses were measured in the Atmospheric and Environmental Chemistry Laboratory of Chemistry Division of the Atomic Energy Centre (AECD), Dhaka and preserved under 4°C temperatures. The aerosol sample having PM2.5 was determined by weighing filter before and after exposure using a micro balance. The difference in weights for each filter was calculated and the mass of each PM2.5 sample thus determined.
Extraction of Samples
The particulate PAHs containing sample was weighed and taken into the volumetric flask, then about 30 ml dichloromethane (DCM solvent solution) was added to dissolve the PAHs, kept for 24 hours then sonicated. After sanitations the extract was filtered through what man filter paper and collected in a clean volumetric flask. Special attention was given to avoid loss of extract. Silica clean up column was prepared and the samples were passed through the column and collected. The total solution was concentrated using liquid nitrogen gas to 1-2 ml and transferred into a GC vial for analysis.
Preparation of standard PAHs solution
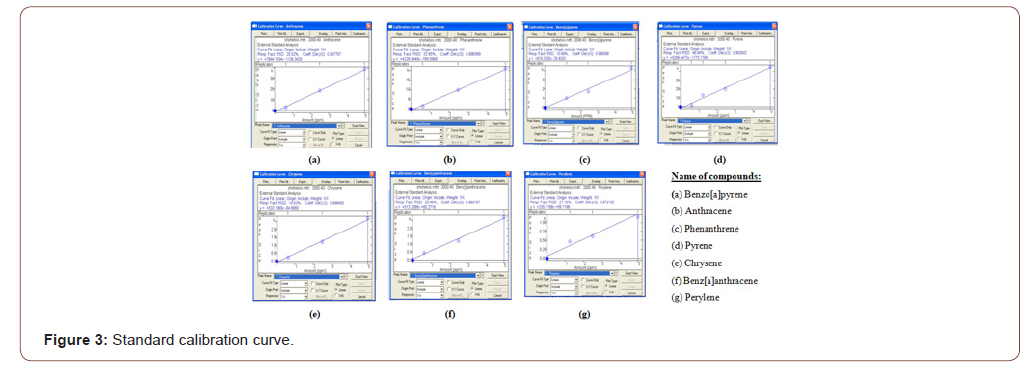
A known amount of PAHs was dissolved in definite amount of solvent (dichloromethane) to prepare 5 ppm PAHs standard solution of Phenanthrene, Anthracene, Pyrene, Chrysene, 1.2-benzanthracene, Perylene, Benzo-a-pyrene, marked with individual identification number and was stored in the refrigerator. The quantitative determination of PAHs has been done by external calibration curve method. The calibration curve of each compound is prepared with known concentrations of the compound prepared and run through GC-MS. Standard curve for each compound is generated by plotting the area vs. the concentration range for corresponding samples. Over this concentration range, the linear regression analysis of peak areas (y) in function of concentration (x), calculated by least square method. Calibration curve for each compound is presented in (Figure 3).
Chromatogram of a standard PAHs solution
The GC column temperature program employed was 400C to 2800C, started from 400C with holding time 1 min and then raised to 1600C at 100C min-1 ramping and finally the temperature raised to 2800C at 150C min-1 ramping. The injector and detector temperature were 2500C and 2800C respectively. The difference in the chemical properties between different molecules in a mixture and their relative affinity for the stationary phase of the column will promote separation of the molecules as the sample travels the length of the column. The molecules are retained by the column and then elute come off from the column at different time (called the retention time), and this allows the mass spectrometer downstream to capture, ionize, accelerate, deflect, and detect the ionized molecules separately. The mass spectrometer does this by breaking each molecule into ionized fragments and detecting these fragments using their mass-to-charge ratio. So, the components have been separated and detected through their retention time and quantified the area through their charge to mass ratio. The retention time of standard solution is 21.59, 21.75, 25.79, 29.40, 29.51, 33.23 and 33.45 min for Phenanthrene, Anthracene, Pyrene, Chrysene, Benz[a]anthracene, Perylene, Benzo[a]pyrene respectively.
Result and Discussion
Analysis of different PAHs in PM2.5 samples
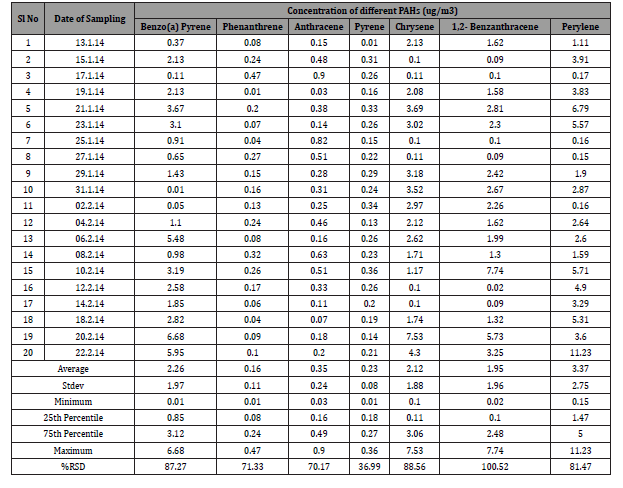
Distribution of different PAHs revealed that the concentrations vary from time to time depending on the trend of air flow. In general, concentration of total PAHs is easily affected by location and seasonal variation. Besides local sources of PAHs, in both urban and rural areas, transport of PAHs through atmosphere can play a large role. The highly carcinogenic benzo[a]pyrene was normally found in the range of 1-20 ng/m3 in Europe, and around 1 ng/m3 in the USA. For other PAHs, individual concentrations were generally in the range of 1-50 ng/m3 in Europe, 0.1-1 in North and South America and in Australia, 1-10 in Japan, and 10-100 in two towns in India and New Zealand [17]. The measured concentration of Phenanthrene, Anthracene, Pyrene, Chrysene, 1,2- Benzanthracene, Perylene, Benzo-a-Pyrene are presented in (Table 1).
Table 1: Concentration of Different PAH’s in air samples collected from Gazipur, Dhaka District, Bangladesh.
Benz [a] anthracene
Benz [a] anthracene or benzo [a] anthracene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12. According to scientists, more than 20% of the carbon in the universe may be associated with PAHs, possible starting materials for the formation of life. Benzo [a] anthracene is a constituent of tobacco smoke [17]. There was a dose-dependent decrease in cell density was observed due to exposure of benz (a)anthracene.
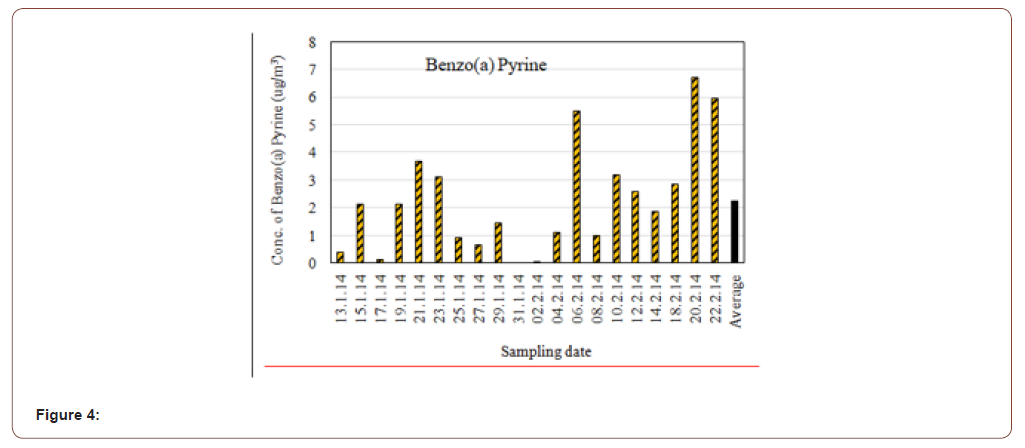
In the present study the average concentration of Benzo(a) pyrene in the atmospheric particulate matter (PM2.5) was found 2.26μg/m3. The highest concentration was found 6.682μg/m3 and lowest concentration was 0.009μg/m3 (Figure 4). The strongly carcinogenic benzo[a]pyrene was typically found in the range of 1–20 ng/m3 in Europe and around 1 ng/m3 in the USA [18]. Present study reveals the mean concentration of Benzo[a]pyrene is 2.269 μg/m3, which is almost 22 times more from India and 100 times more than from Europe [18] and which may be the result of incomplete combustion of organic matter at temperatures. The other sources may be residential wood burning, automobile exhaust fumes (especially from diesel engines) as the sample location is within a heavy traffic area. [19] Reported, after long-term inhalation of “pure” Benzo [a] pyrene at a concentration of 10ng/m3, cancer of the respiratory tract occurred in 35% of golden hamsters. The range of unit lifetime risks calculated from a number of selected Benzo[a]pyrene studies included in a meta-analysis was 1.1 × 10-3 to 4.8 ×10-3μg/m3. Some other risk estimates of respiratory tract cancer related to Benzo[a] pyrene in the ambient air have been calculated by the US Environmental Protection Agency and the estimated risk per year ranged from 0.11 × 10-5 to 1.4 × 10-5 per ng of Benzo[a]pyrene per m3(EPA Report No. EPA-450/5-83-006, 1984).
Phenanthrene
Phenanthrene is composed of three fused benzene rings and in its pure form, it is found in cigarette smoke, it is a known irritant, photosensitizing skin to light. Phenanthrene appears as a white powder having blue fluorescence. Phenanthrene is the backbone of morphinan, which in turn is the backbone of a large number of psychoactive chemicals including antitussives, analgesics, and dissociative drugs. Phenanthrene is absorbed readily from the gut and lungs. In general, these PAHs are highly lipid-soluble and pass across epithelial membranes.
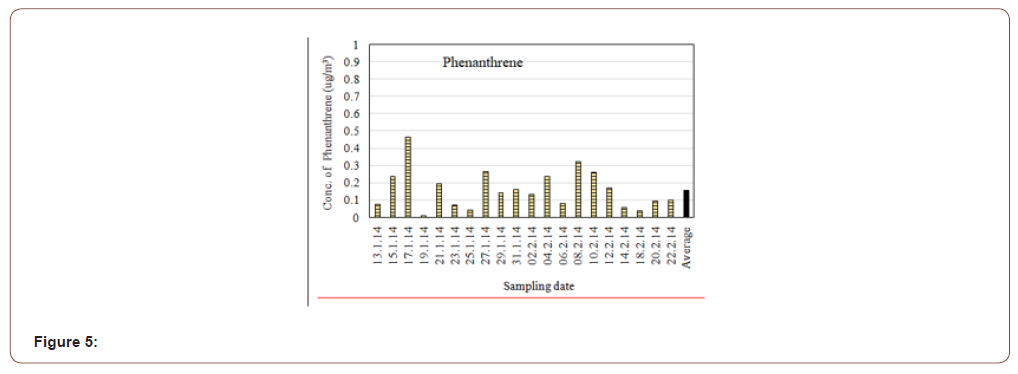
In this study, the average concentration of Phenanthrene in the atmospheric particulate matters (PM2.5) was found 0.159μg/m3 with a highest concentration of 0.465μg/m3 and lowest of 0.039μg/ m3 (Figure 5). Phenanthrene is a rather common PAH. It occurs naturally in fossil fuels and is a product of incomplete combustion. The primary emission sources of phenanthrene are the combustion of fossil fuels but in this case it may be from traffic and exhausts from industry.
Anthracene
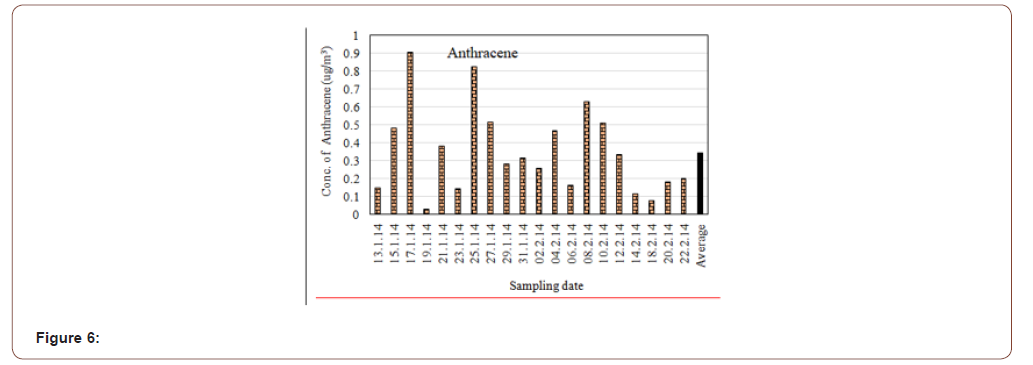
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. Coal tar, which contains around 1.5% anthracene, remains a major source of this material. Anthracene, as many other polycyclic aromatic hydrocarbons, is generated during combustion processes. Exposure to humans mainly happens through tobacco smoke and ingestion of food contaminated with combustion products. Many investigations indicate that Anthracene is noncarcinogenic: “consistently negative findings in numerous in vitro and in vivo genotoxicity tests”. Furthermore, it is readily biodegraded in soil. It is especially susceptible to degradation in the presence of light [20]. The average concentration of Anthracene in the Gazipur air was detected 0.345μg/m3. The highest concentration was 0.903μg/m3 and lowest concentration was 0.025μg/m3 (Figure 6). The vast majority of anthracene is released to the environment when combustion is incomplete (usually because there is insufficient oxygen). In this case the possible source is emission from vehicle exhausts and domestic wood and coal fires. Emissions also arise from industrial effluents, municipal wastewater treatment facilities, waste incinerators and aluminum smelting.
Pyrene
Pyrene is a PAH with a molecular formula C16H10 and made up of four fused benzene rings, which results in a flat aromatic system. It is a colorless solid which is the smallest peri-fused PAH (one where the rings are fused through more than one face). It is formed during incomplete combustion of organic compounds. Pyrene was first isolated from coal tar, where it occurs up to 2% by weight [21]. Therefore, it is produced in a wide range of combustion conditions. Although it is not as problematic as benzopyrene, animal studies have shown that pyrene is toxic to the kidneys and the liver [21].
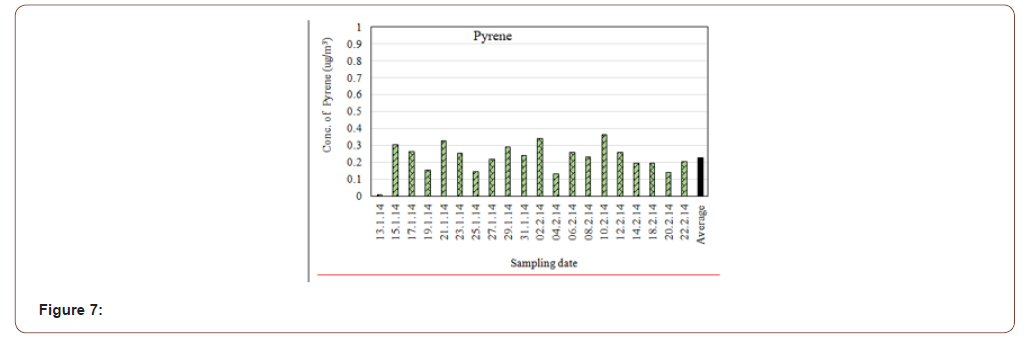
The average concentration of Pyrene was found in the gazipur air (PM2.5) was 0.227μg/m3. The highest concentration was 0.364μg/m3 and lowest concentration was 0.011μg/m3 (Figure 7). It is released to the environment through various waste streams. Some source of pyrene includes exhaust from motor vehicles, cigeratte smoke, coal, oil and wood burning furnaces. It is produced in a wide range of combustion conditions. For example, automobiles produce about 1μg/km [20].
Chrysene
Chrysene is a PAH with the molecular formula C18H12 and made up of four fused benzene rings. It is a natural constituent of coal tar, from which it was first isolated and characterized. It is also found in creosote, which is a chemical used to preserve wood. Chrysene is formed in small amounts during the burning or distillation of coal, crude oil and plant material [22]. However, high purity chrysene is colorless, the yellow color being due to the traces of its yellow-orange isomer tetracene, which cannot be separated easily [22]. Chrysene is a ubiquitous environmental contaminant that occurs as a product of the incomplete combustion of organic compound. Humans are exposed to chrysene by oral, inhalation, and dermal routes. Exposure occurs through the consumption of fruits and vegetables grown in areas with high soil or atmospheric concentrations of chrysene and from drinking or using water contaminated with chrysene. Meats, particularly those with high fat contents, contribute significant quantities of chrysene to the diet from the paralysis of fats during the cooking process. Foods smoked or cooked over open coals contain even greater concentrations.
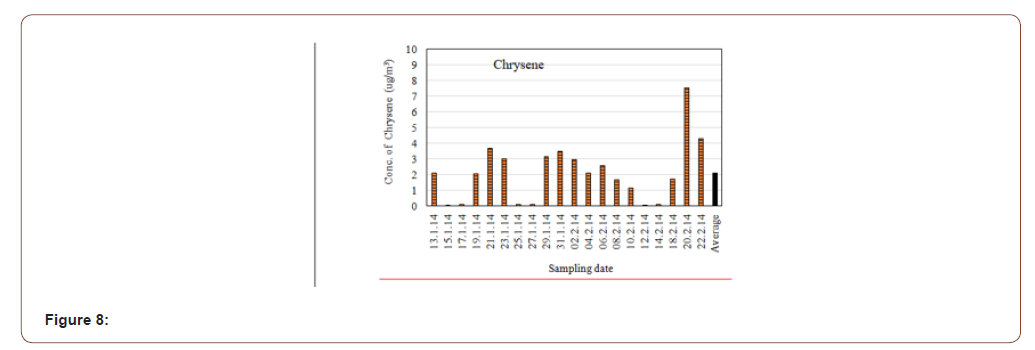
In this study the average concentration of Chrysene in the atmospheric particulate matters (PM2.5) was found 2.120μg/m3. The highest concentration was 7.533μg/m3 and lowest concentration was 0.100μg/m3 (Figure 8). It is a natural constituent of coal tar. Chrysene is estimated to have only about 1% of the toxicity of benzopyrene. It is also found in creosote at levels of 0.5-6 mg/kg [23].
1, 2-Benzanthracene
1, 2-Benzanthracene is a PAH with a molecular formula C18H12, is available as colorless to yellow brown fluorescent flakes or powder. It is stable, combustible, and incompatible with strong oxidizing agents. On decomposition, 1,2-benzanthracene releases carbon monoxide, carbon dioxide, acrid smoke, and fumes. During work, 1,2-benzanthracene can be absorbed into the body of occupational workers by inhalation, through the skin, and by ingestion. Exposures may cause irritation to the eyes, skin, and respiratory tract. Exposures to 1,2-benzanthracene is known to cause kidney damage.
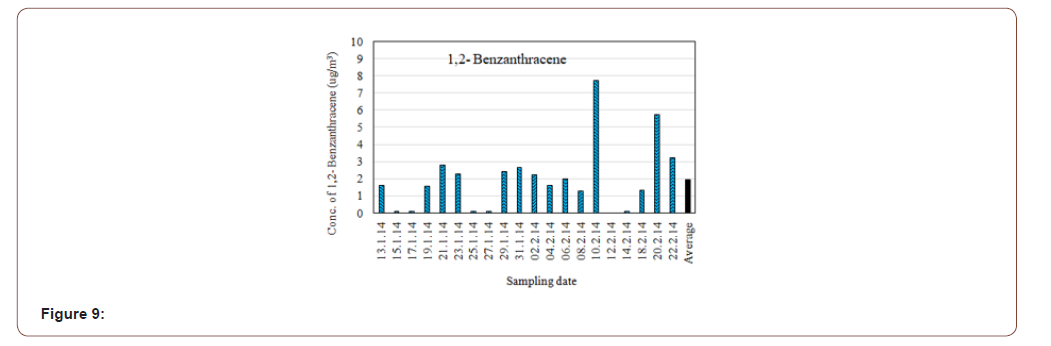
The average concentration of 1,2-Benzanthracene was found in the atmospheric particulate matters (PM2.5) 1.954 μg/m3. The highest concentration was 7.736μg/m3 and lowest concentration was 0.019μg/m3 (Figure 9). The major source may be gasoline and diesel exhaust. Benz (a) anthracene is a carcinogenic constituent of tobacco smoke [24].
Perylene
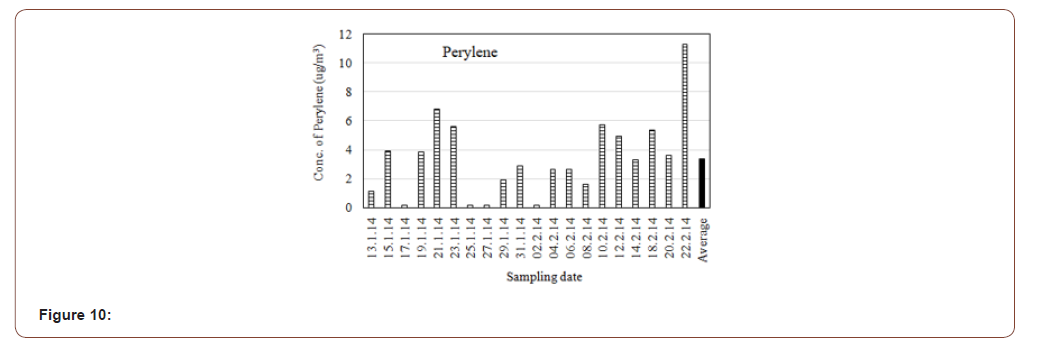
Perylene or perilene is a polycyclic aromatic hydrocarbon with the chemical formula C20H12, occurring as a brown solid. It or its derivatives may be carcinogenic, and it is considered to be a hazardous pollutant. In cell membrane ecyto chemistry, perylene is used as a fluorescent lipid probe. Perylene are formed during the incomplete burning of coal, oil, gas, wood, garbage, or other organic substances, such as tobacco and charbroiled meat. Perylene did not induce mutations in cultured human lymphoblastic cells. Perylene exerted a cytotoxic effect on human keratinocytes in vitro. The agent is not classifiable as to its carcinogenicity to humans. The average concentration of Perylene in the present study was found 3.374μg/m3. The highest concentration was 11.229μg/m3 and lowest concentration was 0.155μg/m3 (Figure 10).
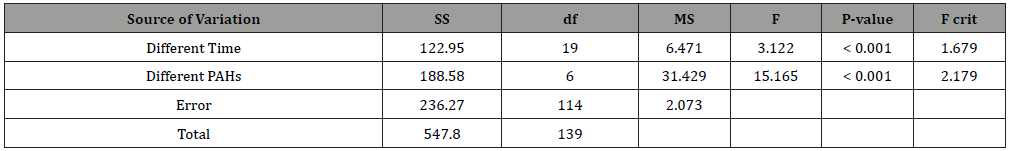
ANOVA test also revealed (Table 2) that the concentration of different PAHs species are significantly different (Fcal> F crit) at different sampling times, which indicated that concentration of PAHs species are correlated with times and seasons. Two-way ANOVA test revealed that the concentration of different PAHs species (Fcal> F crit) are significantly different from each other at a 95% confidence level. ANOVA test also revealed that the concentration of different PAHs species are significantly different (Fcal> F crit) at different sampling times, which indicated that concentration of PAHs species are correlated with times and seasons.
Table 2: Two-way ANOVA.
Pearson’s Correlation
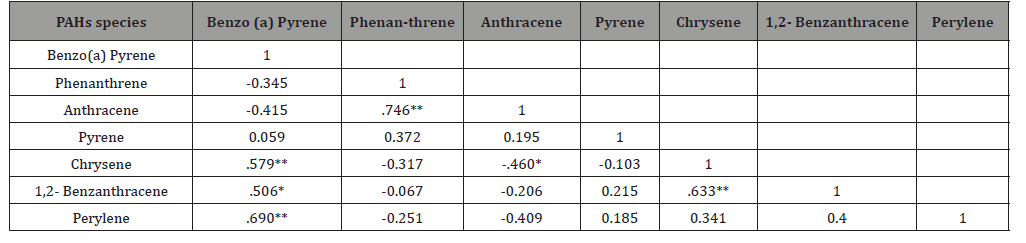
The Pearson’s correlation matrix between all the variables (e.g. Benzo(a)Pyrene, Phenanthrene, Anthracene, Pyrene, Chrysene, 1,2- Benzanthracene and Perylene) in air particulate matter PM2.5 samples was performed using a windows version software ‘IBM SPSS Statistics 20’ (IBM, USA); and the results are presented in (Table 3). The study revealed a significant positive correlation between the Chrysene and Benzo (a) Pyrene, 1,2-Benzanthracene and Perylene; Benzo (a) Pyrene and Perylene; Anthracene and Phenan-threne; and 1,2- Benzanthracene and Chrysene at a 95% Confidence level -indicating they are coming from the same source/s. Reversely a negative correlation was also observed between Chrysene and Anthracene (-0.460*) at a 95% confidence level.
Table 2: Pearsons correlation.
Conclusion
The average concentration of Benzo-a-Pyrene, Phenanthrene. Anthracene, Pyrene, Chrysene, 1,2- Benzanthracene and Perylene, were 2.26μg/m3 , 0.16μg/m3, 0.35μg/m3 , 0.23μg/m3 , 2.12μg/ m3 , 1.95μg/m3 , and 3.37μg/m3 respectively The most carcinogenic Benzo-a-Pyrene (BAP) was detected with a concentration of 2.26μg/m3 in Gazipur air but WHO guide line value for BAP is between 0.1 and 1.3 ng/m3 for Lung cancer. The strongly carcinogenic benzo[a]pyrene was typically found in the range of 1–20ng/ m3 in Europe and around 1ng/m3 in the USA but in our study it is 2.26μg/m3 which is almost 100 times more than that found in Europe and USA. Average concentration of seven PAHs of Gazipur area was 1.49μg/m3. Whereas the average concentrations of particle- bound PAHs were 0.27±0.16μg/m3 in North Chinese Plain and hence Gazipur air contains more than 5 times greater amount of PAH’s and is really alarming [25]. showed that average concentration of measured sixteen polycyclic aromatic hydrocarbons (PAHs) in total suspended particulate matter (TSPM) from an urban and industrial cum residential site in Agra (India) from December 2005 to December 2006 were 115±17ngm3 [26]. Also reported, average concentration of total PAH was extremely high, with annual average concentration of 155ng/m3 in air sample collected from the Kathmandu Valley in the foothills of the Himalayas. The average concentrations of particle-bound total PAHs were 267ng/m3 in North Chinese Plain. [27] And hence in the present study average total PAHs are 10.439μg/m3.
Due to the industrialization and modernization of society, the introduction of motorized vehicles, and the explosion of the human population, massive traffic, burning of fuel, burning of coal in the brick kilns and power plant, burning of different types of plastic, dust particles from the different construction sites were the sources of the PAHs in the Gazipur area. The concentration was found in a level where there is a potential threat to carcinogenic effect due to long time exposure. Therefore, it is essential to develop an air pollution abatement strategy to protect people from the hazardous effects arising from elevated atmospheric PAHs by the systematic study of air pollution. The Government of Bangladesh should take immediate action to reduce these high concentrations of PAHs [28].
Acknowledgement
The authors would like to express their thanks and gratitude to Atmospheric and Environmental Chemistry laboratory, Atomic Energy Centre, Dhaka and Chemistry Department of University of Dhaka for the collaboration to carry out this research work. The authors also like to acknowledge the authority of air monitoring station (CAMS-4) site of Clean Air and Sustainable Environment (CASE) project, Gazipur for their assistance to collect the air particulate matter samples.
Conflict of Interest
Author declares no conflict of interest.
References
- Ravindra K, Sokhi R, Van Grieken R (2008) Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmospheric Environment 42(13): 2895-2921.
- Nekhavhambe TJ (2008) Determination and distribution of polyaromatic hydrocarbons in rivers, surface runoff and sediments in and around Thohoyandou, Limpopo Region, Thesis University of Venda p. 1-9.
- Abdel Shafy Hussein I (2016) A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum 25(1): 107-123.
- Tobiszewski M, Namieśnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environmental Pollution 162: 110-119.
- Nielsen T (1984) Reactivity of polycyclic aromatic hydrocarbons towards nitrating species. Environ Sci Technol 18(3): 157-163.
- Kamens RM, Guo Z, Fulcher JN, Bell DA (1988) Influence of humidity, sunlight, and temperature on the daytime decay of polyaromatic hydrocarbons on atmospheric soot particles. Environmental Science and Technology 22: 103-108.
- Bostrom CE, Gerde P, Hanberg A, Jernstrom B, Johansson C, et al. (2002) Cancer Risk Assessment, Indicators and Guidelines for Polycyclic Aromatic Hydrocarbon in The Ambient Air. Environ Health Perspect 110 (Suppl 3): 451-488.
- Baird WM, Hooven LA, Mahadevan B (2015) Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action, Environ Mol Mutagen 45(2-3): 106-114.
- Slaga TJ (1984) Multistage skin carcinogenesis: A useful model for the study of the chemoprevention of cancer. Acta Pharmacol Toxicol (Copenh) 55(2): 107-124.
- Neff JM (1979) Polycyclic aromatic hydrocarbon in the aquatic environment: sources, fates and biological effects. Applied Science Publisher Ltd, Essex, UK Pp. 262.
- Lee D, Hansen, Delbert, Eatough j (1991) Organic Chemistry of the Atmosphere (Telford Press). CRC; 0849388341.
- Pitts JN, Sweetman JA, Zielinska B, Winer AM, Atkinson R (1985) Determination of 2-nitrofluoranthene and 2-nitropyrene in ambient particulate organic matter: Evidence for atmospheric reactions. Atmospheric Environment 19(10): 1601-1608.
- Kunzli N, Tager I (2005) Air pollution: from lung to heart. Swiss Medical Weekly 135 (47-48): 697-702.
- Ridker PM (2009) C-Reactive Protein: Eighty Years from Discovery to Emergence as a Major Risk Marker for Cardiovascular Disease. Clinical Chemistry 55(2): 209-215.
- Rossner P Jr, Sram RJ (2012) Immunochemical detection of oxidatively damaged DNA. Free Radic Res 46(4): 492-522.
- Adkonis M, Wolska L, Przyjazny L, Namieśnik J (2006) Sources of errors associated with the determination of PAHs and PCBs analytes in water sample. Taylor & Francis Group 39: 2317-2331.
- Lerner JEC, Sanchez EY, Sambeth JE, Porta AA (2012) Characterization and health risk assessment of VOCs in occupational environments in Buenos Aires, Argentina. Atmospheric Environment 55: 440-447.
- Menichini E (1992) Urban air pollution by polycyclic aromatic hydrocarbons: levels and sources of variability. Science of the Total Environment 116(1-2): 109-135.
- AlPauluhn J (1985) Long-term inhalation study with benzo(a)pyrene and SO2 in Syrian golden hamsters. Ex pathol 28(1): 31.
- Senkan, Selim Castaldi, Marco (2003) Combustion, in Ullmann’s Encyclopedia of Industrial Chemistrt, Wiley-VCH, Weinheim.
- (2005) Basic Concepts of Environmental Chemistry. (2nd Ed) Des W Connell Taylor and Francis/CRS press, Boca Raton, P. 480.
- Soehl A (2012) Assessment of Benzo-alpha-pyrene Emissions in the Great Lakes Region. International Emission Inventory Conference pp. 23-24.
- Anjasören Sen, Bodo Wichert (2009) Asphalt and Bitumen in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim.
- Talhout Reinskje, Schulz Thomas, Florek (2011) Hazardous Compounds in Tobacco Smoke. Int J Environ Res Public Health 8(12): 613-628.
- Rajput N, Lakhani A (2014) Measurements of polycyclic aromatic hydrocarbons in an urban atmosphere of Agra, India. Environmental Pollution 192: 83e90.
- Chen P, Kang S, Li C, Rupakheti M, Yan F, et al. (2015) Characteristics of Polycyclic Aromatic Hydrocarbons in Atmospheric aerosols in the Kathmandu Valley, Nepal. Science of the total environment 538: 86-92.
- Liu S, Tao S, Liu W, Dou H, Liu Y, et al. (2008) Seasonal and spatial occurrence and distribution of atmospheric polycyclic aromatic hydrocarbons (PAHs) in rural and urban areas of the North Chinese Plain. Environ Pollution 156(3): 651-656.
- (1984) Review and evaluation of the evidence for cancer associated with air pollution. Arlington, VA, US Environmental Protection Agency.
-
YN Jolly, A Tabassum, MA Rahman, MS Rahman. Contamination Status of Polycyclic Aromatic Hydrocarbons (PAH s) In Atmospheric Particulate Matter PM2.5 Samples of a Semi-Residential Area of Dhaka, Bangladesh. 3(2): 2020. GJNFS.MS.ID.000560.
-
Polycyclic Aromatic Hydrocarbon, Air-Metrics Mini-Vol Samplers, Gas Chromatography-Mass Spectrometry, Quartz Filters, Wildfires, Ubiquitous, Combustion, Extraction, Aromatic Hydrocarbons, Carcinogenic, Atherogenesis
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






