 Research Article
Research Article
Effect of Feeding Spirulina platensis and Trigonella foenum-gracecum Seed as Functional Feed Ingredients on Growth Performance and Oxidative Stability in Turkey Meat
Farhana Sharmin1, Md Sazedul Karim Sarker1,2*, Md Abdur Rashid2 and Shakila Faruque2
1Strengthening of Poultry Research and Development Project, Bangladesh Livestock Research Institute, Bangladesh
2Poultry Production Research Division, Bangladesh Livestock Research Institute, Savar, Dhaka, Bangladesh
Md. Sazedul Karim Sarker, Strengthening of Poultry Research and Development Project, Bangladesh Livestock Research Institute, Bangladesh
Received Date:January 26, 2022; Published Date:April 18, 2022
Abstract
This experiment was conducted to investigate the effect of feeding Spirulina platensis and Trigonella foenum-gracecu (Fenugreek) seed as functional feed ingredients on growth performance and oxidative stability in turkey meat. A total of one hundred sixty (160) 7 days old turkey poult were split into five dietary treatments, with four replications having twenty poults in each group. The five dietary treatments were formulated using basal feed as follows: control (T1), including 2 different levels of fenugreek seed 0.5% (T2) and fenugreek seed 1%, (T3), S. platensis 1.5% + fenugreek seed 0.5% (T4) and S. platensis 1.5% + Fenugreek seed 1%, (T5). Turkey poults were reared up to 70 days of age and fed ad libitum. There were no statistically significant differences on feed intake and average daily gain however body weight at 70 days were significantly deference in T5 group. The feed conversion ratio was slightly lower in the additives group than control. The lowest (p≤0.05) thiobarbituric acid-reactive substances values (TBARS) of breast and thigh were obtained in T2-T5 groups compared to T1 group after the second week of preservation. The serum and meat cholesterol were also significantly deference in all additives group. The results of the present study elucidated that dietary inclusion of the S. platensis, fenugreek could be promising functional ingredients to produce value-added turkey meat in terms of oxidative stability and lowering cholesterol in meat.
Keyword:Turkey; Spirulina platensis; Fenugreek; Cholesterol; Lipid oxidation
Introduction
Turkey is a type of large poultry species originated in North America. Nowadays USA, France, Italy, Russia, Germany, Poland, Morocco and Portugal are the main producers of turkey meat in the world [1]. Turkey production and turkey meat processing are the fast-growing branch of poultry production. The interest to turkey meat is continuously increasing during the last decades because its delicate taste and texture [2]. Turkey meat is the second most consumed poultry meat worldwide for its modest levels of total lipids, saturated fatty acids (SFA), and cholesterol content [3-5]. Its meat is popular due to highly nutritive properties, hypoallergenic, and safe [6]. Amirkhanov, et al. [7] reported that turkey meat is rich in B group vitamins, which help to prevent anemia maintain the normal functioning of the cardiovascular and nervous system. Apart from these nutrients, it also contains zinc and an excellent source of high protein. The biological value of poultry protein is defined by its content of essential amino acids and ratio, as well as its digestibility [8]. The quality composition of amino acids of poultry was described by Sokolov, et al. [9] and Essary and Ritchey (1968), is being slightly richer in lysine and arginine than beef [10]. However, the chemical composition of poultry meat differs according to the age and breed [11]. The main difference between turkey and other poultry is its large size, high breeding performance and yield of edible parts; turkeys sur- pass other birds in live mass. Recently Zampiga, et al. [3] reported that the need for extending knowledge not only on broiler chickens but also on turkeys because the growing concern of the poultry in- dustry regarding these new emerging meat quality issues.
At present, consumers are very much conscious about their health and they are willing to pay more for natural, safe, and eco-friendly food products. For these reasons the demands of functional or value-added food are increasing.
The feeding and rearing conditions under which the poultry are produced and slaughtered may also influence the meat’s oxidative stability. Lipid oxidation is a primary cause of quality deterioration in meat products through adverse changes in undesirable odors and flavors, which lowers the functional, sensory, and nutritive values of meat products. Therefore, there is a need to increase the antioxidation capacity of meat that can be achieved by addition of natural product in the diet. Fenugreek (Trigonella foenum graecum) is an annual plant belongs to the family Leguminosae. The interest in fenugreek seed was prompted by its widely and numerous uses as a spice and herbal remedy particularly for lowering cholesterol levels [12] antibacterial, antidiabetic, hepatoprotective effect and also it has anticancer activity [13]. The bioactive compounds of fenugreek such as fiber, saponins and other phytonutrients including phenolic acids and flavonoids. These bioactive components have responsible of its antioxidant activity, hypo-cholesterol emic activity, and lactation aid. On the other hands, till to date, seaweeds are also a staple food of many countries. Global demand for macroalgal and microalgal foods is growing, and algae are increasingly being consumed for functional benefits beyond the traditional considerations of nutrition and health [14]. Spirulina platensis has recently served as an important source of valuable bioactive compounds. Dried spirulina is a good nutritional source with high protein and significant poly- unsaturated fatty acids content, such as oleic, linoleic, gamma-lin- olenic, and docosahexaenoic (DHA) acid [15]. Keeping the above facts in consideration, the current study was therefore designed to examine the effect of different dietary levels of fenugreek seeds and S platensis and their combination on growth performance, choles- terol and oxidative stability in brown turkey meat in Bangladesh.
Materials and Methods
Preparation of S platensis and fenugreek seed meal
The blue-green algae S. platensis was collected from Bangladesh Council of Scientific and Industrial Research (BCSIR) in Dhaka, Bangladesh. On the other hand, fenugreek seed was collected from local marker in Savar, Dhaka, Bangladesh and subsequently grinded into powder using a milling machine to pass through a 0.15-mm sieve, then tightly packed in polythene plastic bags, sealed and kept at room temperature until required. S. platensis and fenugreek seed were analyzed in triplicate for crude protein (CP), ether extract (EE), moisture and ash as described by Association of Official Analytical Chemists [16] data was presented as supplementary information S1.1
Experimental design, dietary treatments and bird management
A total of 160 turkey poult 7 day old were taken for this study. Their parents were collected from local source as a foundation stock. The chicks were randomly weighed and allocated to 20 floor pens (100 cm long × 90 cm wide) in shed containing rice husk at a depth of 5 cm. The internal temperature of the turkey poult house was set and maintained at 34°C for the first week, after which it was gradually reduced to 23°C at 3°C per week, and then maintained at this temperature until the end of the total experimental period. The experiment was divided into five dietary treatments, with 4 repli- cations having 8 poults in each dietary group. Each pen served as an experimental unit. The poults were vaccinated against commercial Newcastle Disease Virus (NDV) through eye drops and drinking water at days 4 and 18 during the experiment period, respectively. The poults were inspected daily, and dead birds were removed following mortality recording (pen, date, and body weight [BW]). Feed and fresh water were offered ad libitum feed intake and free access to water throughout the experimental period (70 d rearing). The birds’ were reared following standard management practices and basal diet was formulated to meet the Nutrient Requirements of Poultry and applied for a total of 10 weeks in two stages: starter (0-3 weeks) and grower (4-10 weeks), the diet and chemical com- position of additives are shown in supplemental information. All diets were formulated as mash form at the feed formulation room of poultry division of BLRI, Savar, Dhaka. Five dietary treatment groups were produced from the basal feed as follows: control (T1), including fenugreek seed 0.5% (T2) and fenugreek seed 1%, (T3), S. platensis 1.5% + fenugreek seed 0.5% (T4) and S. platensis 1.5% + Fenugreek seed 1%, (T5). A proximate component analysis was per- formed for analyzing moisture, crude protein, ash, and ether extract of the experimental diets according to the Association of Official Analytical Chemists’ [16] methods. The diets’ metabolizable energy (ME) contents were calculated on the basis of the determined nu- trients in the proximate analyses. The BW per pen were recorded at the trial premises, at weekly intervals (i.e., up to 70 d of age). The BW (g) was calculated as final body weight (ΔBW) minus initial BW; and body weight gains (BWG, g) were calculated accordingly. The feed intake (FI, g) was calculated as feed allocated minus feed refused; whereas, the feed conversion ratio (FCR) was calculated as the FI (g) per ΔBW (g) on a pen weight basis. Experimental birds were reared in the research shed at Bangladesh Livestock Research Institute Savar, Dhaka. The experiment was also subjected to an assessment of turkey health status for its ethical acceptability and was approved by the Ethics Committees of Bangladesh Livestock Research Institute in Savar, Dhaka.
Slaughter procedure
Forty turkey poults consisting eight birds were randomly selected per treatment, 2 per replicate pen, and were individually weighed at the age of 70 d. Birds were then fasted for 8 h with water offered ad libitum consumption and were reweighed before they were slaughtered. After bleeding, scalding, plucking, and washing, the feet, head, neck and skin were removed. Then, the carcasses were manually eviscerated and cut into breast, drumsticks (legs), wings, and thighs. The visceral organs and cuts were then weighed individually, and the yields and carcass dressing percentage (CW/BW) were calculated. The breast and thigh muscles were separated from the bones and skin and was trimmed of external/adjacent fat and connective tissue. The breast and thigh meat samples from each poult were ground separately using a meat grinder. The samples were subsequently divided into three parts, one for the oxidative stability analysis and another two for the proximate and cholesterol composition analysis. Finally, the samples were kept into zipper bag, after which those for oxidative rancidity analysis were refrigerated at 4°C and samples for other analyses were stored at -20°C.
Determination of oxidative stability
The lipid oxidation of the turkey meat was determined accord- ing to the method described by Sarker, et al. [17], with slight mod- ification. For this analysis, 4 g of the thigh and breast meat sample were blended at full speed for 1.5 min in a chilled stainless watering blender cup with 10 mL of extracting solution containing 20% tri- chloroacetic acid (TCA) in 2M phosphoric acid. The resulting sedi- ment was quantitatively transferred to a 50 mL conical tube with 10 mL of distilled water, homogenized and diluted by shaking. After, the aliquot was filtered through Whatman No.6 filter paper, then 5 mL of filtrate was transferred to a test tube, and 5 mL of 2-thio- barbituric acid (0.005 M in DW) was added. The solution was then subsequently shaken in a water bath at 80°C (HB-205 SW Hanbaek Scientific Co., Korea) for 30 min. After cooling, the color develop- ment was measured at 530 nm in a Jenway 6305 Spectrophotom- eter (Bibby Scientific Ltd., Staffordshire, United Kingdom). TBARS values were expressed as micromoles of malondialdehyde (MDA) per 100 g of meat sample.
Determination of cholesterol
At the end of the experimental period, three birds were ran- domly selected from each replicated pen for a lipid profile analysis to be performed. Blood samples were carefully collected from the selected birds’ brachial vein and quickly transferred to centrifuge tubes. Care was taken to avoid any contamination or disturbance. The blood samples were then centrifuged for 15 min at 1500 g in a cold chamber at 4°C. The sera were then carefully removed, trans- ferred to plastic vials and stored at-20°C until such time as they were analysed for total cholesterol, total triglyceride, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cho- lesterol (LDL-C). The lipid profiles were carried out using a Snibe Automated Biochemical Analyzer Biossays BC1200.
Meat cholesterol was extracted according to the method de- scribed by Sharmin, et al. [18] with minor modifications. Five grams of meat sample was weighed in extracting tubes to which a solution of 10 mL of pyrogallol (6% in ethanol, w/v) and 1.0 ml of 5α-cholestane (Sigma Aldrich, MO, USA) was added as an internal standard (1mg/ml). After sonication for 10 min, 5.0 mL of 60% po- tassium hydroxide in de-ionised water was added. The extracting tube was heated at 80°C for 1 h in a shaking water bath at 90 rpm (ST30, Nuve, Turkey). After cooling, 15 ml of 2% sodium chloride in de-ionised water and 15 mL of extracting solvent (hexane: ethyl acetate = 85:15, v/v) containing 0.01% BHT (Butylated hydroxytol- uene) were added and mixed vigorously for approximately 2 min. The tubes were left undisturbed for 10 min, after which the upper layer was collected into a 50-mL volumetric flask. This extraction step was repeated two more times; extracts were combined and passed through anhydrous sodium sulphate, which acted as a filter to remove water. The volume of the extracts was made up to 50 mL with extracting solvent. A 10 mL aliquot of the extract was dried under N2 gas and re-dissolved in 1.0 mL n-hexane (purity 95%). Ex- tracts were filtered through a 0.25 μm membrane filter (Advantec, PTFE, Duran Germany) and analysed using gas chromatography (GC). Cholesterol separation was conducted using an Agilent 8890 Series (Agilent technology, USA) GC system equipped with a HP-5 capillary column (30mm × 0.32mm, 0.25 μm, Agilent Technologies, Santa Clara, CA, USA) and a flame ionisation detector (FID) (H2: 30 mL/min, air: 300 mL/min). The flow rate of the carrier gas (N2) was 3 mL/min. Injection and detection temperatures were both 260°C. Cholesterol was separated on a column set at 250°C for 5 min and elevated to 260°C at a rate of 5°C/min. The column temperature was reduced to 250°C at a rate of 5°C/min and maintained for 2 min before the next injection. Cholesterol data processing was carried out with Open Lab CDS software, and peaks were identified by com- parison with retention times of standards. the cholesterol standard was purchased from TCI (Tokyo, Japan) and used for cholesterol quantification. A 1μL aliquot was injected into the GC column. Con- centrations were calculated by comparing peak areas with those obtained from the standard solutions and were expressed as mg /100 g cholesterol in meat.
Statistical analysis
The analytical measurements were done in triplicates, and the results were presented as the average of 3 analyses ± the standard deviation (SD). The statistical analysis was done using the SPSS sta- tistical package (IBM Corp., IBM SPSS Statistics for Windows, Ver- sion 16.0, Armork, NY, USA) with a one-way ANOVA. A p value of < 0.05 was taken as statistically significant based on Duncan’s tests.
results
Turkey’s growth performance and meat composition
The effects of diets with added S platensis and fenugreek on the FI, ΔBW, and FCR are shown in (Table 1). The dietary addition of fenugreek and S. platensis affected the turkey’s growth perfor- mance. The final ΔBW and BW gain were significantly higher (p < 0.05) in the T2 and T6 groups compared to the T1, control group. Similarly, on d 70, birds fed diets with S. platensis and fenugreek combination in particular those in T2 to T6 had observed decreased feed consumption ratio as compared to control (T1) group of turkey for the total period. No mortality was observed in the experimental period.
Addition of feed additives had an effect on the composition of breast and thigh meats (Table 2). Dietary addition of fenugreek and S. platensis groups were increased (p<0.05) crude protein content in breast meat compared to the control group. In the breast meat crude fat content was increased in T6 group whereas decreased in other groups. Crude protein content did not differ with control group; but there was found higher moisture content in the T5 group compared to other group (p<0.05) in respect of thigh meat. The ether extract content in thigh meat was slightly lower in additives group than the control group T1 (1.62%); however crude ash con- tent did not significantly differ both thigh and breast meat. Results showed that heart, liver, and small intestine weight were not signifi-cantly different (p<0.05) among the treatments (Table 3). However, breast meat was significantly differed between the additives and control group in this study. Abdominal fat pad was not observed both the additives and control group.
Table 1:Effect of feeding S. platensis and fenugreek as feed additives on body weight, average daily gain of turkey.
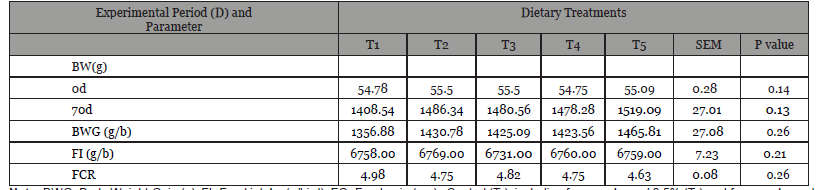
Note: BWG- Body Weight Gain (g), FI- Feed intake (g/bird), FG- Feed:gain (g:g) ; Control (T1), including fenugreek seed 0.5% (T2) and fenugreek seed 1%, (T3), S. platensis 1.5% + fenugreek seed 0.5% (T4) and S. platensis 1.5% + Fenugreek seed 1%, (T5).
Table 2:Analyzed chemical composition (% dry matter)) of turkey meat.
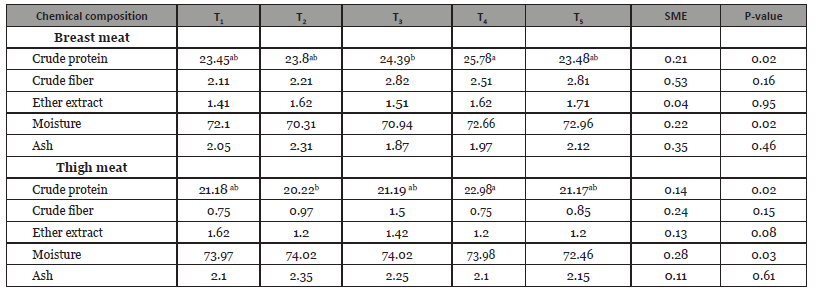
Note: Control (T1), including fenugreek seed 0.5% (T2) and fenugreek seed 1%, (T3), S. platensis 1.5% + fenugreek seed 0.5% (T4) and S. platensis 1.5% + Fenugreek seed 1%, (T5).
Table 3:Effect of S. platensis and fenugreek as natural feed additives on internal cut of turkey.
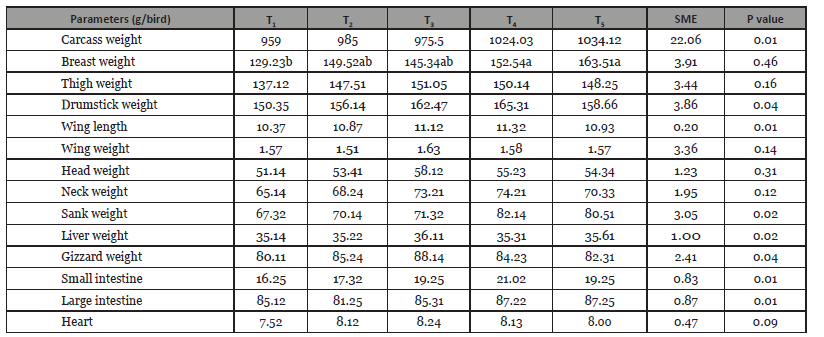
Note: Control (T1), including fenugreek seed 0.5% (T2) and fenugreek seed 1%, (T3), S. platensis 1.5% + fenugreek seed 0.5% (T4) and S. platensis 1.5% + Fenugreek seed 1%, (T5).
Oxidative stability of broiler meat
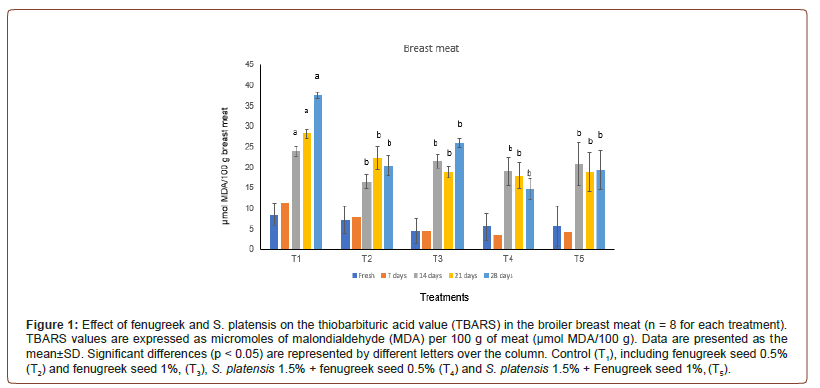
The TBARS test, of broiler breast and thigh meats which de- termine the amount of malondialdehyde (MDA), a major second- ary lipid oxidation byproduct was shown in Figure 1 and Figure2. Additives groups had lower (p<0.05) TBARS values after wks 1 and 2 of preservation as well as 3rd wks values in breast and thigh meat compare to control group, T1. Significantly lower TBARS val- ues were observed in breast meat of turkey fed diet with additives fenugreek and S. platensis groups from d 7 to d 28 compared to the control group. Dietary addition of fenugreek and S. platensis groups significantly reduced the TBARS values of in thigh meat in all mea- surement periods (p<0.05). At d 28, the reduction in breast meat TBARS value was significant high compared to thigh meat in control group (37.63 and 47.77 μmol MDA/100 g respectively) whereas, at d 28 it was significantly lower (p<0.05) value obtained in all ad- ditive’s groups of the breast and thigh meat samples compared to control (Figure 1, 2).
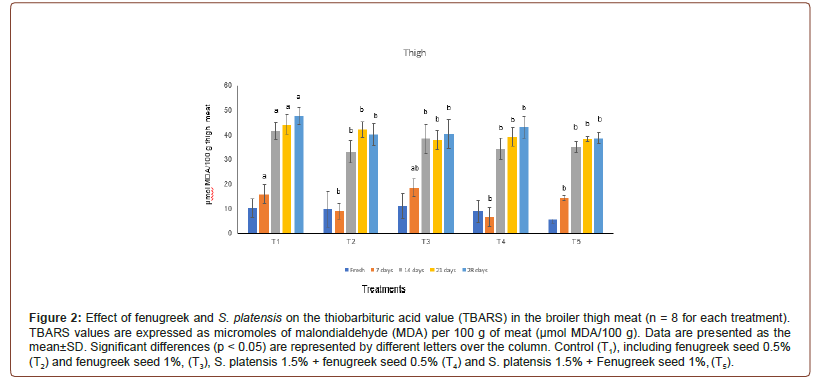
Cholesterol content in turkey meat
The cholesterol content was determined from turkey serum and meat sample and are shown in (Table 4). Blood lipid profile has an important role in the performance and carcass quality of turkey. Feed additives groups showed the lowest cholesterol values compared to control. It was interesting that T3 and T4 had lower in total serum cholesterol 126.33 and 127.13mg/dl, respectively compared to T1 group (153.31mg/dl). In addition, high density li- poprotein cholesterol (HDL-C) was found significantly higher in T2 and T5 groups that is fenugreek seed 0.5% and S. platensis 1.5% + fenugreek seed 0.5% with the values of 63.50 and 67.50 mg/dL,respectively whereas T1 had 60.67 mg/dL. Addition of fenugreek and S. platensis were significantly reduced LDL cholesterol (T2, T3 and T6 with values of 69.50, 65.25 and 67.75 mg/dL) compared to control group, T1 (85.67 mg/dL). Triglyceride in serum had signifi- cantly reduced after addition of S. platensis 1.5% and fenugreek 1% in fed compared to T1, group. In addition, there was no significantly deference in meat cholesterol.
Table 4:Effect of S. platensis and fenugreek as natural feed additives on serum lipid profile level of turkey.
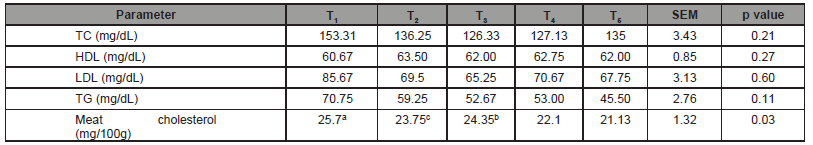
Note: TC- Total cholesterol; HDL – High density lipoprotein; LDL- Low density lipoprotein; TG- Triglyceride; Control (T1), including fenugreek seed 0.5% (T2) and fenugreek seed 1%, (T3), S. platensis 1.5% + fenugreek seed 0.5% (T4) and S. platensis 1.5% + Fenugreek seed 1%, (T5).
Discussion
Data presented in Table 4 showed that the effect of treatments on final weight and weight gain of turkey was significantly deference in the S. platensis and fenugreek addition group and control. In case of FCR that was slightly lower in additives group but the differences were not significant than control. It should be mentioned that a low FCR means bird gain more weight when eating less feed. Common- ly, BW is used to monitor animals’ nutritional status and growth [19]. Numerous studies have shown that S. platensis dietary addi- tion can improve the growth performance of poultry [20]. However, very few study was reported about dietary addition of S. platensis and fenugreek on turkey meat. The level of feed consumption is a basic and important factor that determines the rate of growth and body composition achieved by animals throughout their life cycles. These results are in agreement with those of previous researchers, who recorded nonsignificant effects of dietary S. platensis addition on performance parameters [21]. In summary, it can be stated that natural additives have great potential, with the right combination and doses..
The results of the proximate analyses of turkey meat (white meat) are presented in Table 5. The highest protein content deter- mined in turkey breast meat than thigh meat. Differential charac- teristic of poultry meat is a high ratio of protein (more complete protein, and less hardly digestible, noncomplete proteins, like colla- gen and elastin), which determine its high nutritive value [22]. Gasi- lina [23] examined the chemical composition of commercial and backyard turkey meat and showed that the protein content of white meat varied from 18.89% in commercial to 22.37% in backyard turkeys. Moreover, they reported their turkey meat samples con- tained 2.44% (commercial) and 2.76% (backyard) fat and 1.08% (commercial) and 1.04% (backyard) ash. However, in our research it was observed that in breast meat fat contain 1.41~1.71% and in thigh meat 1.20~ 1.62%. Benkova, et al. [24] established that based on the age the protein contain could be varied such as in breast muscle protein content of 24.90% on Ivagal turkeys at 17 weeks of age, and 23.60% at 16 weeks of age. Fat content of breast muscle of male birds was 0.94%, and of females-1.04%. The respective values of thigh meat were 0.79% in female and 1.34% in male turkeys. Iva-nov, et al. [25], reported that the protein, fat and ash content in tur- key meat varied from 24-25%, 0.3-1.0% and 1.2-1.6%, respectively. Hence, Turkey meat has favorable dietary nutrition, especially for elderly people. While the white meat contains more protein and less water than red meat.
Lipid oxidation causes the loss of nutritional and sensory values as well as the formation of potentially toxic compounds that com- promise meat quality and reduce shelf life. Enhancing the antioxi- dant capability in muscle tends to improve meat quality and extend shelf life [26]. The extent of lipid oxidation of raw turkey breast meat, as measured by MDA formation, was significantly different among the fresh and 28 days of preservation time in both thigh and breast meat. No differences occurred in fresh breast and thigh meat samples. The increase of TBARS values during refrigerated storage of turkey meat shows that oxidative deterioration started from the 7th day and was increased until the 28th day of storage. Comparison of the malondialdehyde (MDA) per 100 g of meat values found in Samouris, et al. [27] study shows that turkey meat is more sensitive to oxidative deterioration than chicken meat. The difference has been attributed primarily to the weaker ability of turkeys to store dietary tocopherol in their tissues compared to chickens [28,29]. The antioxidant capacity of poultry meat depends largely on the concentration of the contained α-tocopherol, which in turn is de- pendent on the level of α-tocopheryl acetate added to the diet [30]. Spirulina contains many substances such as pigments (for example carotenoids such as β-carotene and zeaxanthin) [31], phycobilipro- teins vitamins [32], macro and micro mineral elements [32,33] and antioxidants [34]. Fenugreek also contain saponins and flavonoids. The less oxidation in the additives group might be responsible their bioactive component.
The serum lipid and meat cholesterol of the turkey was also fa- vorably altered by fenugreek and Spirulina added to feed (Table 4). A highly significant (p < 0.05) reduction in serum cholesterol lev- els was noted upon feeding with fenugreek and Spirulina added to the diet, which may be due to hypo-cholesterol emic effect of these two components. It was reported from the that decreasing plasma cholesterol level in turkey by addition of fenugreek and S. platensis groups in the diet, which indirectly revealed about the hypo-choles terol emic effect of feed additives group. Among the additive groups,in Poultry Meat Processing and Quality. GC Mead, ed. Woodhead Publis fenugreek seed 1%, T3 and T4, S. platensis 1.5% were found to be most effective in reducing serum cholesterol levels in turkey. The hypolipidemic effect is due to the high saponin content in fenugreek seed found saponin to be the most active anti-nutritional substance in these leaves [35]. Since plant saponin binds with cholesterol, as mentioned above, it can be expected that saponin reduces the level of cholesterol in the body. Eskandar, et al. [36] also reported that some saponins can prevent hypercholesterolemia, a phenomenon that results from complex formation with cholesterol. Complex for- mation between cholesterol and plant saponin has been investigat- ed for the past few years [37]. According to Patil, et al. [38] [39-41], the reduction of cholesterol and triglycerides by alkaloids is partly due to a reduction in lipogenic enzyme activity and an increase in the excretion of bile acids in the faces.
Conclusion
The results obtained in this study revealed that the addition of different levels of fenugreek and Spirulina platensis and their combination in the turkey poult’s diets had no effect on WG, FI, FCR and internal organ. Cholesterol content especially total meat cholesterol was significantly decreased in the additives group of T3 and T4 diet group however final body weight was significantly increased in T5 group. Furthermore, the current study results also demonstrated that the addition of fenugreek, and combination of fenugreek and Spirulina platensis could reduce TBARS value. Therefore, it can be concluded that diets having fenugreek and S. platensis could be used as feed additives in turkeys’ diets. However, further follow-up research is required to know the mechanisms of various pathways of reducing cholesterol and enrichment of ω-3 fatty or amino acids content in the meat from different turkey varieties.
Acknowledgments
The author(s) disclosed receipt of the following financial sup- port for the research, authorship, and/or publication of this article. This work has been funded by the Strengthening of Poultry Research and Development Project, Bangladesh Livestock Research Institute (BLRI), Savar, Dhaka-1341, Bangladesh under the Ministry of Livestock and Fisheries, Bangladesh.
Conflict of Interests
The author(s) declare no conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Aksenova KN, Kagadiy VV, Prischepa TS, Patiyeva AM, Manuylova TP (2015) Physical and chemical parameters of meat of broad breasted white turkeys. Young Scientist 12: 111-115.
- Oblakova M, Ribarski S, Oblakov N, Hristakieva P (2016) Chemical composition and quality of turkeybroiler meat from crosses of layer light (ll) and meat heavy (mh) TURKEY. T J S 2: 142-147.
- Zampiga M, Tavaniello S, Soglia F, Petracci M, Mazzoni M, et al. (2019) Comparison of 2 commercial turkey hybrids: productivity, occurrence of breast myopathies, and meat quality properties. Poult Sci 98(5): 2305-2315.
- Remignon H (2004) Production of turkeys, geese, ducks and game birds in Poultry Meat Processing and Quality. GC Mead, ed. Woodhead Publishing Duxford UK. Pp: 211-231.
- USDA (2011) Nutrition Facts, Chicken and Turkey, USDA Food Safety and Inspection Service.
- Lisitsyn AB, Semenova AA, Kuznetsova TG, Dydykin AS, Nasonova VV (2018) Study of the effect of sex and type of muscles on the development of quality defects in Turkey meat after the slaughter. Foods Raw Mater 6 (1): 63-70.
- Amirkhanov K, Igenbayev A, Nurgazezova A, Okuskhanova E, Kassymov S, et al. (2017) Comparative analysis of red and white turkey meat quality. Pakistan J Nut 16(6): 412-416.
- Jukna V, Klementavičiūtė J, Meškinytė-Kaušilienė E, Pečiulaitienė N, Samborskytė M, et al. (2012) Comparative evaluation of quality and composition of ostrich, turkey and broiler meat. Biotechnol Anim Hus 28 (2): 385-392.
- Sokolov AA, Pavlov DV, Bolshakov AS (1970) Technology of Meat and Meat Products. Pishevaya Promyshlennost Moscow.
- Ahmad S, Ahsan H, Yousaf M, Kamran Z, Ataur R, et al. (2013) Effect of feeding whole linseed as a source of polyunsaturated fatty acids on performance and egg characteristics of laying hens kept at high ambient temperature. Braz J Poult Sci 15 (1): 21-25.
- Davleev AD (2004) Mechanically deboned poultry meat. Alfa-Dizain (In Russian).
- Basu Sk, Thomas JE, Acharya SN (2007) Prospects for growth in global nutraceutical and functional food markets: a Canadian perspective. Aust J Basic Sci 1: 637-649.
- Sajad Ahmad Wani, Pradyuman Kumar (2018) Fenugreek: A review on its nutraceutical properties and utilization in various food products. J Saudi Soc Agric Sci 17(2): 97-106.
- Mark LW, Philippe P, James SC, John AR, Sabeeha SM, et al. (2017) Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol 29(2): 949-982.
- Ciferri O (1983) Spirulina, the edible microorganism. Microbiol Rev 47(4): 551-578.
- AOAC (2000) Official methods of analysis of the association of Official analytical chemists. (17th), Gaithersburg MD, USA.
- Sarker MSK, Ko SY, Lee SM, Kim GM, Choi JK, (2010) Effect of different feed additives on growth performance and blood profiles of Korean Hanwoo calves. Asian-Aust J Anim Sci 23(1): 52-60.
- Sharmin F, Jeong BG, Jung JY, Chun JY (2017) Cholesterol analysis of Korean eat-out foods for national food composition database. J Food Sci Technol 54(7): 1837-1849.
- Nkukwana TT, Muchenje V, Pieterse E, Masika PJ, Mabusela TP, et al. (2014) Effect of Moringa oleifera leaf meal on growth performance, apparent digestibility, digestive organ size and carcass yield in broiler chickens. Live Sci 161: 139-146.
- Zahroojian N, Moravej H, Shivazad M (2013) Effects of dietary marine algae (Spirulina platensis) on egg quality and production performance of laying hens. J Agric Sci Technol 15: 1353-1360.
- Ross E, Dominy W (1990) The nutritional value of dehydrated, blue-green algae (Spirulina platensis) for poultry. Poult Sci 69(5): 794-800.
- Okuskhanova E, Smolnikova F, Kassymov S, Zinina O, Mustafayeva A, et al. (2010) Development of minced meatball composition for the population from unfavorable ecological regions. ARRB 713(3): 1-9.
- Gasilina VA (2012) Veterinary-sanitary expertise of commercial and backyard turkey meat from Krasnoyarsk region. PhD Thesis Department of Veterinary Siberian Federal University Krasnoyarsk Moscow.
- Benkova J, Lukacka J (1995) Chemical composition of breast and thigh muscular subctance in turkey- cocks and turkey hens of Ivagal breed at the age of 16 and 17 weeks. J Anim Sci 28: 233-241.
- Ivanov S, Pasichniy V, Strashynskyi I, Marynin A, Fursik O, et al. (2014) Meat products from turkey meat with texture-forming fillers. Food Chem Technol (Maisto Chemija ir Technologija) 48: 25-33.
- Barbut S (2002) Poultry Meat Processing and Product Technology. Poultry Products Processing: An Industry Guide. CRC Press, Boca Raton FL Pp: 1-30.
- Samouris GI, Bampidis VA, Sossidou E, Zantopoulos N (2006) Lipid oxidation of raw and cooked turkey breast meat during refrigerated storage. Arch Geflügelk 71 (1): 41-44.
- Sklan D, Bartov I, Hurwitz S (1982) Tocopherol absorption and metabolism in the chick and turkey. J Nutr 112(7): 1394-1400.
- Wen J, Mccarthy SN, Higgins FM, Morrissey PA, Buckley DJ, et al. (1997) Effect of dietary α-tocopherol acetate on the uptake and distribution of α-tocopherol in turkey tissues and lipid stability. Irish J Agric Food Res 36: 65-74.
- Papageorgiou G, Botsoglou N, Govaris A, Giannenas I, Iliadis S, et al. (2003) Effect of dietary oregano oil and a-tocopheryl acetate supplementation on iron-induced lipid oxidation of turkey breast, thigh, liver and heart tissues. J Anim Physiol Anim Nutr 87(9): 324-335.
- Maoka T (2011) Carotenoids in marine animals. Mar Drugs 9(2): 278-293.
- Becker EW (1994) Nutrition. In: Microalgae: Biotechnology and Microbiology. Cambridge University Press Cambridge. pp. 196-249.
- Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101: 87-96.
- Christaki E, Bonos E, Giannenas I, Florou-Paneri P (2013) Functional properties of carotenoids originating from algae. J Sci Food Agric 93(1): 5-11.
- Mamoun T, Mukhtar MA, Tabid MH (2014) Effect of fenugreek seed powder on the performance, carcass characteristics and some blood serum attribute. Adv Res Agri Vet Sci 1: 6-11.
- Eskandar M, Hossein K, Shahrzad P, Somayeh H (2015) In-vitro Evaluation of Complex Forming Affinity of Total Saponin Extracted from Ziziphus spina-christi and Quillaja saponaria with cholesterol. RJPBCS 6: 619-623.
- Sharmin F, Koyama T, Koyama H, Ishizaki S (2020) Cholesterol-binding ability of saponin from Japanese Starfish. J Food Sci Technol 58(8): 3056-3064.
- Patil RH, Prakash K, Maheshwari VL (2010) Hypolipidemic effect of Celastrus paniculatus in experimentally induced hypercholesterolemic Wistar rats. Indian J Clin Biochem 25(4): 405-410.
- Bou R, Guardiola F, Tres A, Barroeta AC, Codony R (2004) Effect of dietary fish oil, a-tocopheryl acetate, and zinc supplementation on the composition and consumer acceptability of chicken meat. Poult Sci 83(2): 282-292.
- Jung S, Choe JH, Kim B, Yun H, Kruk ZA, et al. (2010) Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci 86(2): 520-526.
- NRC (National Research Council) (1980): Recommended Dietary Allowances. (9th), Washington DC. 1980: 26.
-
Farhana Sharmin, Md Sazedul Karim Sarker, Md Abdur Rashid and Shakila Faruque. Effect of Feeding Spirulina platensis, Fenugreek Seed as a Functional Feed Ingredient on Growth Performance and Oxidative Stability in Turkey Meat. Glob J Nutri Food Sci. 3(5): 2022. GJNFS.MS.ID.000573.
-
Thio-barbituric acid-reactive substances values, Saturated fatty acids, Hypo-cholesterol emic activity, Antioxidant activity, Docosahexaenoic, Gamma-linolenic, Ether extract, Crude protein, Newcastle Disease Virus
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






