 Research Article
Research Article
Association of Immunoglobulin E Levels with Smoking and Trace Element Intake: A Cross-sectional Analysis of the Shika Study
Koji Katano1, Fumihiko Suzuki2,3, Hiromasa Tsujiguchi1,2,4, Akinori Hara1,2,4, Sakae Miyagi5, Takayuki Kannon4,6,Keita Suzuki2, Yukari Shimizu7, Thao Thi Thu Nguyen8, Kim-Oanh Pham1, Atsushi Asai1, Tomoko Kasahara1,Masaharu Nakamura2, Chie Takazawa1, Koichiro Hayashi1, Toshio Hamagishi2, Aki Shibata2, Tadashi Konoshita9,Yasuhiro Kambayashi10, Hirohito Tsuboi11, Atsushi Tajima4,6, Takayuki Kobayashi2 and Hiroyuki Nakamura1,2,4*
1Graduate School of Medical Science, Kanazawa University, Japan
2Department of Hygiene and Public Health, Kanazawa University, Japan
3Community Medicine Support Dentistry, Ohu University Hospital, Japan
4Advanced Preventive Medical Sciences Research Center, Kanazawa University, Japan
5Innovative Clinical Research Center, Kanazawa University, Japan
6Department of Bioinformatics and Genomics, Kanazawa University, Japan
7Department of Nursing, Komatsu University, Japan
8Faculty of Public Health, Haiphong University of Medicine and Pharmacy, Vietnam
9Third Department of Internal Medicine, University of Fukui Faculty of Medical Sciences, Japan
10Department of Public Health, Okayama University of Science, Japan
11Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Japan
Hiroyuki Nakamura, Department of Hygiene and Public Health, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa, Japan.
Received Date:March 04, 2022; Published Date:March 16, 2022
Abstract
Introduction: Although many epidemiological studies have investigated the relationship between smoking and immunoglobulin E (IgE)
levels, few have reported a relationship with the intake of trace elements. Therefore, this epidemiological study examined the relationship between
smoking and IgE antibody titers, with the factor of trace element intake, in middle-aged and elderly inhabitants living in Shika town, Ishikawa, Japan.
Methods: Participants comprised 771 inhabitants of Shika town and included 363 males and 408 females with mean ages of 61.93 years
and 62.13 years, respectively. Non-specific IgE was obtained from the medical checkups. Smoking status was asked using the questionnaire. Trace
element intakes were investigated using the brief-type self-administered diet history questionnaire.
Results: Trace elements that showed interactions in a two-way analysis of covariance were copper and manganese. A post hoc analysis using
the Bonferroni method demonstrated that copper intake was significantly higher in the low-IgE group than in the high-IgE group of current smokers
only. A multiple logistic regression analysis showed that copper and manganese significantly contributed to higher IgE levels in current smokers only.
Conclusions: Our cross-sectional study of middle-aged and elderly community residents, among males, revealed lower manganese and
copper intakes in the high-IgE group of current smokers only, suggesting that IgE levels are more easily increased by allergens in smokers with low
manganese and copper intakes.
Keyword:Cigarette smoking; Serum immunoglobulin; Copper; Manganese; Cross-sectional study
Abbreviations:ANCOVA-Analysis of covariance; BDHQ-Brief-type self-administered Diet History Questionnaire; BMI-Body Mass Index; CIConfidence Interval; IgE-Immunoglobulin E; OR-Odds Ratio; SD-Standard Deviation
Introduction
Although the prevalence of allergic diseases, such as bronchial asthma, allergic rhinitis, atopic dermatitis, and food allergy, is approximately 20% [1,2], it has rapidly increased in recent decades [3,4]. The causative agents of allergic diseases are allergens, such as pollen, microorganisms, dust mites, house dust, or pets. Exposure to airborne particulates, such as environmental chemicals and tobacco, may also exacerbate allergic reactions [3]. In recent years, increases in these chemicals in the environment have been suggested to aggravate allergic diseases [5]. Since most cases of allergic rhinitis and asthma are genetically predisposed to produce immunoglobulin E (IgE) antibodies to common environmental allergens, allergy is known as an IgE-mediated disease [6].
Tobacco tar or polycyclic aromatic hydrocarbons [7,8], which are involved in allergic reactions, may increase the number of memory B cells, which are the source of IgE production, through the effects of nicotinic receptors, including the alpha4 and alpha7 subunits, in B cell lines [9], and exacerbate the IgE-mediated symptoms of allergic rhinitis, such as sneezing, itching, nasal congestion, and rhinorrhea [10]. Epidemiological studies reported a dose-dependent relationship between the number of cigarettes smoked and IgE antibody titers [11] and demonstrated that IgE levels increased with not only active smoking, but also passive smoking [12]. On the other hand, a cross-sectional study on untreated patients diagnosed with allergic rhinitis showed that nasal obstruction in patients with moderate to severe symptoms and quality of life due to rhinitis did not significantly differ between smokers and non-smokers [13]. Therefore, the relationship between smoking and IgE levels remains unclear.
Although few epidemiological studies have investigated the relationship between trace element intake and IgE levels, Tsuji, et al. [14] reported a negative correlation between animal dander-specific IgE and blood manganese levels in pregnant women. Furthermore, Oluwole et al. [15] revealed that serum concentrations of manganese negatively correlated with asthma in schoolchildren. A clinical study on healthy adults also demonstrated that a low-copper diet increased the percentage of circulating B cells, the source of IgE production [16]. This mechanism is supported by findings showing a marked increase in B lymphocytes in the spleens of copperdeficient rats [17]. Although adequate intakes of manganese and copper may inhibit increases in IgE antibodies and B cells, which produce IgE antibodies, the findings of epidemiological studies have been inconclusive. Combining the above-mentioned previous studies, we hypothesized that smokers who have insufficient intake of the trace elements elevate IgE antibody which is related to various allergic diseases. Therefore, this epidemiological study investigated the association of immunoglobulin E levels with and without smoking and trace elements intake in middle-aged and elderly inhabitants.
Materials and Methods
Participants
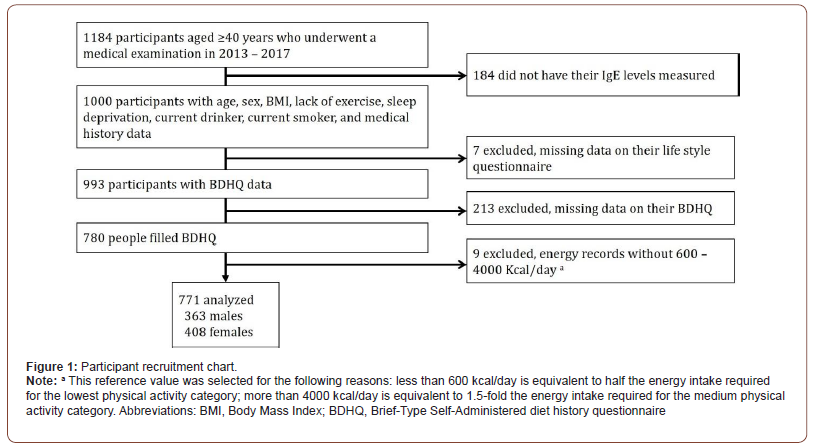
We utilized cross-sectional data from the Shika study [18-20]. Participants were recruited between October 2013 and January 2017. The target population was residents living in 4 model districts (Horimatsu, Higashimasuho, Tsuchida, and Togi districts) in Shika town, Ishikawa prefecture, Japan (population, 21,061, population aged 65 years and older on 1 September 2020, 8499 (aging rate 42.2%)) [21]. The 4 districts were randomly selected, and approximately 50% of the population of the town live in these districts. A total of 5013 residents aged 40 years and older live in the model districts. Written informed consent was obtained from all 5013 participants. Among the participants who underwent a medical examination, 1184 agreed to take part in this study. Among the participants who underwent a medical examination, 1184 agreed to take part in this study. Four hundred and thirty patients were excluded for the following reasons: IgE levels were not examined, the questionnaire was not answered, or energy records were not within 600–4000 Kcal on the brief-type self-administered diet history questionnaire (BDHQ). Figure 1 shows inclusion criteria. In total, 771 participants (363 males and 408 females with mean (SD) ages of 61.93 (11.01) years and 62.13 (11.07) years, respectively) were included in the analysis. No one received the treatment with anti-IgE antibodies (Figure 1).
IgE levels
Blood samples were collected during medical checkups in the Shika study mostly from December to January annually. Nonspecific IgE levels were measured by SRL Inc. (Tokyo, Japan) using a Phadia5000 instrument (Thermo Fisher Scientific/Phadia AB, Uppsala, Sweden) according to the manufacturer’s instructions.
BDHQ
Trace elements were assessed using the BDHQ [22,23]. The BDHQ is a four-page structured questionnaire that assesses the consumption frequencies of 58 foods and beverages commonly consumed by the general Japanese population. The BDHQ also estimates dietary intake in the last month using an ad hoc computer algorithm. The validity of the BDHQ has been demonstrated in previous studies [22,23]. To analyze nutrient data, the density method was employed for estimations of intake per 1000 Kcal.
Questionnaire on demographics
Participants completed a self-administered questionnaire on their lifestyle and medical history, including age, sex, BMI, lack of exercise, sleep deprivation, current drinker, and current smoker.
Statistical analysis
Participants were classified as current and non-smokers. The two IgE groups were classified into the low- and high-IgE groups based on the median of participants in the present study. [24] IBM SPSS Statistics version 25 for Windows (IBM, Armonk, NY, USA) was used for statistical analyses. The Student’s t-test was performed to examine relationships between continuous variables, while the chisquared test was conducted to investigate relationships between categorical variables. A two-way analysis of covariance (ANCOVA) was performed to examine the main effects and interactions between the two smoking groups and two IgE groups on trace element intake. Adjustments were performed for the following confounding factors: age, lack of exercise, sleep deprivation, current drinker, and BMI. In order to confirm the trace elements that showed an interaction between the two smoking groups and the two IgE groups, we performed multiple logistic regression analysis stratified by smoking status with IgE as the dependent variable. The forced input method was used for variable selection. The significance level was set to 5%.
Sample size
We used the free software G-power to calculate the sample size and statistical power. For the F-tests of ANCOVA, the effect size, alpha error probability, power, number of groups, and number of covariates were set to 0.25, 0.05, 0.8, 4, and 5, respectively. The total sample size and actual power were 128 and 0.801, respectively. For the Z-tests for logistic regression, tails, odds ratio, mull hypothesis, alpha error probability, power, X distribution, X parm π were set to Two, 0.2, 0.25, 0.05, 0.8, Binomial, 0.5, respectively. The total sample size and actual power were found to be 124 and 0.800, respectively. Therefore, the sample size of this study was confirmed to be sufficient.
Ethics statement
The present study was conducted with the approval of the Ethics Committee of Kanazawa University (No. 1491). Written informed consent was obtained from all participants prior to participation.
Results
Participant characteristics
Participant characteristics, the smoking status, IgE levels, and trace element intake are shown in Table 1. Among 771 participants, 363 were male and 408 were female with mean (SD) ages of 61.93 (11.01) years and 62.13 (11.07) years, respectively, and no significant sex differences. IgE levels (p < 0.001) was significantly higher in males than in females. Significantly more males than females were current smokers (p < 0.001). On the other hand, the intakes of iron (p < 0.001), zinc (p < 0.001), copper (p < 0.001), and manganese (p < 0.001) were significantly higher in females.
A comparison of IgE levels with and without allergic disease is shown in S1 Table. IgE levels were significantly higher in the 100 participants of any allergic disease group than in the 648 participants of the non-allergic disease group (p < 0.001) and in the 67 participants of the pollinosis group than in the 681 participants of the non-pollinosis group (p = 0.004).
Table 1:Comparison of IgE levels with and without allergic disease.
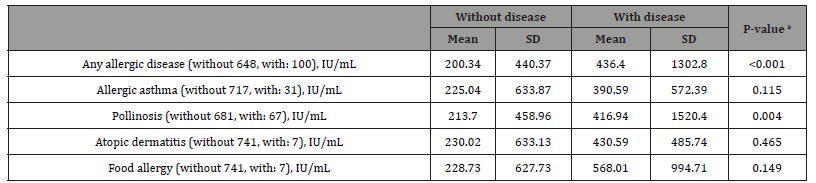
Notes: a One-way analysis of variance. Covariates adjusted for sex and age. Abbreviations: SD, Standard deviation
The mean (SD) number of cigarettes smoked per day among current smokers was 20.35 (11.05) for males and 13.81 (7.72) for females. This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn (Table 1).
Table 1:Participant characteristics.
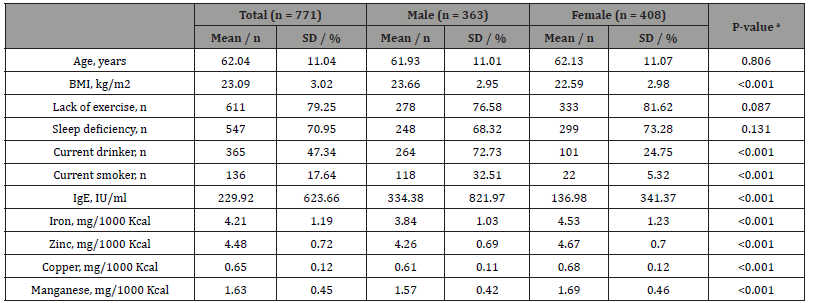
Notes: a p-values were calculated using the Student’s t-test for continuous variables and the chi-squared test for categorical variables. Abbreviations: BMI, body mass index; SD, standard deviation.
Comparison of IgE levels
No significant differences were observed in mean age between the 550 participants in the low-IgE group and the 221 participants in the high-IgE group (62.15 years versus 61.76 years, respectively). The proportion of females (p < 0.001) and the intakes of zinc (p = 0.003), and copper (p = 0.029) were significantly higher in the low- IgE group than in the high-IgE group. In contrast, the proportion of current smokers (p < 0.001) was significantly higher in the high-IgE group than in the low-IgE group (Table 2).
Table 2:Characteristics of low-IgE and high-IgE groups.
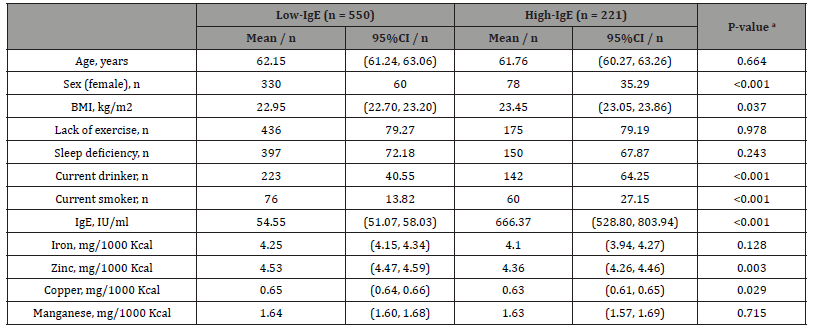
Notes: a p-values were calculated using the Student’s t-test for continuous variables and the chi-squared test for categorical variables. Abbreviations: BMI, body mass index; CI, confidence interval.
Comparison of current smokers
The mean age of 635 non-smokers was significantly higher than that of 136 current smokers (62.94 years versus 57.80 years, p < 0.001). The proportion of females (p < 0.001) and the intakes of iron (p < 0.001), zinc (p = 0.001), and copper (p < 0.001) were significantly higher in non-smokers than in current smokers. In contrast, IgE levels (p = 0.018) was significantly higher in current smokers than in non-smokers. Since there were 110 male smokers and only 26 female smokers, two-way ANCOVA and multiple logistic regression analyses were performed only for the male group (Table 3).
Table 3:Characteristics of non-smoker and current smoker groups.
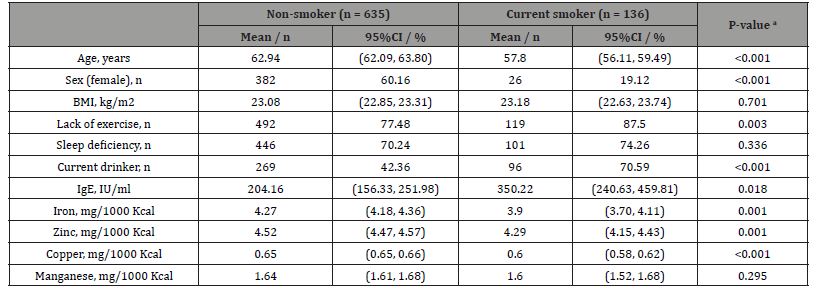
Notes: a p-values were calculated using the Student’s t-test for continuous variables and the chi-squared test for categorical variables. Abbreviations: BMI, body mass index; CI, confidence interval.
ANCOVA between two smoking groups and two IgE groups on trace element intake in males
Table 4 shows the results of a two-way ANCOVA with the two current smoking groups (non-smokers and current smokers) and two IgE groups (low and high) as fixed factors and adjustments for age, BMI, lack of exercise, sleep deprivation, and current drinkers. When 253 non-current smokers were subclassified, 160 were in the low-IgE group and 93 were in the high-IgE group. Similarly, among 110 current smokers, 60 were in the low-IgE group and 50 were in the high-IgE group. The trace element that showed a main effect in the two IgE groups was copper (p = 0.044). The trace elements that showed an interaction between the two groups of current smoking and IgE levels were copper (p = 0.048) and manganese (p = 0.007). Therefore, high intakes of copper and manganese in current smokers appeared to result in low IgE levels (Table 4). In multiple comparisons using the Bonferroni method, copper intake was significantly higher in the low-IgE group than in the high-IgE group within current smokers (Figure 2).
Table 4:Two-way ANCOVA of smoker and IgE groups in males.
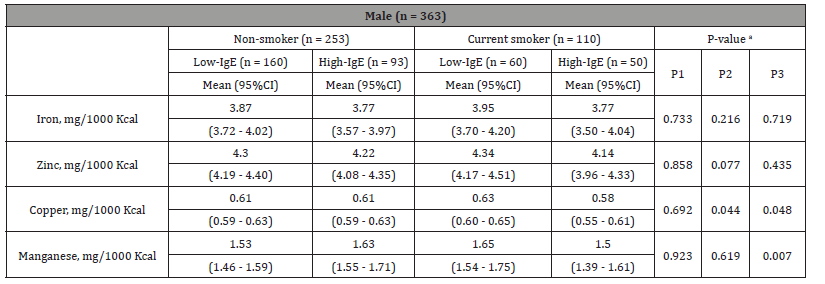
Notes: a Two-way ANCOVA. Adjusted for age, lack of exercise, sleep deprivation, current drinker, and BMI. P1: current smoker, P2: IgE, P3: current smoker × IgE. Abbreviations: CI, confidence interval; ANCOVA, analysis of covariance.
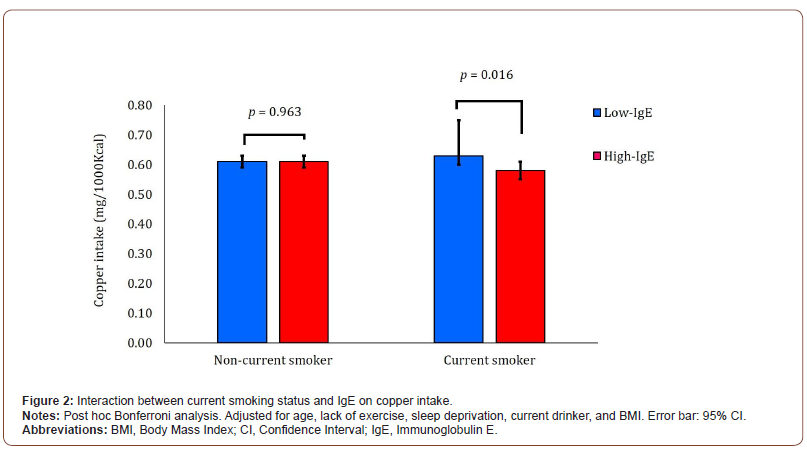
Relationship between trace element intake and IgE levels stratified by the current smoking status in males
The results of a multiple logistic regression analysis stratified by the two groups of current smokers, with IgE levels as the dependent variable, are shown in Table 5. When covariates were adjusted for age, BMI, a lack of exercise, sleep deprivation, and current drinkers, and each independent factor was entered separately, copper (odds ratio (OR) 0.002, 95% confidence interval (CI), ≤ 0.001 - 0.206, p = 0.008) and manganese (OR 0.252, 95% CI, 0.070 - 0.910, p = 0.035) were identified as significant independent variables, whereas none of the trace elements were significant independent variables in nonsmokers. Therefore, similar to the results of the two-way ANCOVA, higher copper and manganese intakes were associated with lower IgE levels in current smokers (Table 5).
Table 5:Relationship between trace elements and IgE levels stratified by current smokers in males.
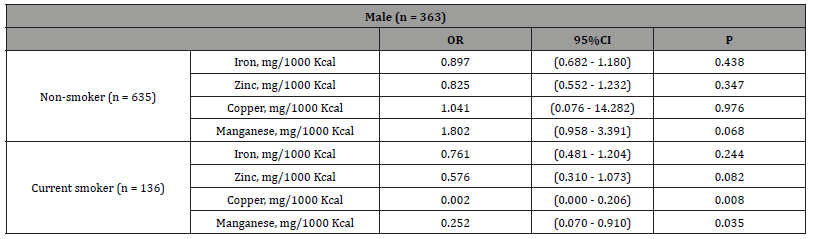
Notes: Adjusted for age, lack of exercise, sleep deficiency, current drinker, and BMI. Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio.
Discussion
The present results showed that IgE levels were significantly higher in current smokers than in non-smokers in the univariate analysis. Furthermore, the percentage of current smokers was significantly higher in the high-IgE group, classified by median IgE levels in participants, than in the low-IgE group. In other words, we found a definite relationship between smoking and IgE levels. In a previous epidemiological study that investigated the relationship between smoking and IgE levels [11], a dose-dependent relationship was observed between smoking and serum IgE levels in a crosssectional analysis of the relationship between urinary cotinine concentrations and serum IgE levels in adult men. A case-control study by Ahmed, et al. [12] revealed increases in IgE levels not only with active smoking, but also with passive smoking. Regarding the mechanism by which smoking increases IgE levels, a review by Qiu, et al. [9] showed that the effects of nicotinic receptors, including the alpha4 and alpha7 subunits, in human B cell lines may increase the number of memory B cells, which are the source of IgE production. The present results support a mechanism by which smoking is involved in IgE production because the percentage of smokers was significantly higher in the high-IgE group than in the low-IgE group.
The prevalence of allergies, such as bronchial asthma [25,26] and cedar pollinosis [27], is higher in smokers than in nonsmokers. A review conducted on the relationship between smoking and asthma by Stapleton et al. [25] showed that smoking was significantly and inversely associated with long-term asthma control and that current smoking is associated with more asthma attacks and nocturnal asthma symptoms. In addition, a crosssectional study by Kim, et al. [26] reported that asthma was also positively associated with e-cigarettes. An epidemiological survey of Japanese cedar pollinosis and smoking status by Tanigawa, et al. [27] indicated that lymphocyte subpopulations in smokers increased more than those in nonsmokers. In the case of cedar pollinosis, smoking is known to exacerbate IgE-mediated allergies [10] based on the correlation between IgE levels and symptoms such as nasal discharge [28,29]. On the other hand, a meta-analysis by Saulyte, et al. [30], which investigated the relationship between smoking and allergic diseases, reported that those associations were very modest in adults, and that the associations were related to passive smoking in allergic rhinitis, active and passive smoking in allergic dermatitis, and indirect smoking in food allergy, respectively. This study also showed that 100 participants with any allergy of pollinosis, allergic asthma, atopic dermatitis, or food allergy, showed significantly higher serum total IgE levels as an indicator of the exacerbation of allergy.
The main results from the two-way ANCOVA and multiple logistic regression analysis in the present study demonstrated that manganese and copper intakes among current smokers were significantly lower in the high-IgE group than in the low-IgE group. A review by Mahfuzur, et al. [31] demonstrated that manganese is a trace element necessary for maintaining good health and is involved in various physiological functions in the body, including defense against oxidative stress, digestion, and immune response. In addition, a review by Wang, et al. [32] demonstrated that the abnormal metabolism of copper, which cannot be produced or synthesized in the body, leads to many diseases, such as impaired immune function, diabetes, coronary heart disease, and osteoporosis. Regarding the relationship between trace elements and IgE levels, a case-control study of 1,562 children by Oluwole, et al. [15] revealed that serum concentrations of manganese negatively correlated with asthma. Furthermore, in a clinical study on 11 healthy men, Kelley, et al. [16] demonstrated that a lowcopper diet increased the percentage of circulating B cells, similar to manganese. This mechanism is supported by the percentage of B lymphocytes in the spleen being significantly increased in experiments on copper-deficient rats by Bala, et al [17]. Therefore, when manganese and copper are sufficiently consumed within the range of appropriate intake, they may suppress increases in IgE level or B cells, the source of its production. Based on these findings, we speculated that IgE levels are elevated by smoking because low manganese and copper intakes did not inhibit IgE production. Although the cessation of smoking effectively suppressed smokinginduced allergies, we consider the appropriate intake of trace elements to be meaningful as health guidance for those with difficulty quitting smoking.
Regarding the limitations of the present study, firstly, due to its cross-sectional nature, it was not possible to elucidate the causal relationship between the intake of trace elements and smoking on IgE production. Secondly, since the intakes of manganese and copper were based on a questionnaire, we did not directly measure their blood levels. Thirdly, participants were volunteers; therefore, the possibility of selection bias needs to be considered. Fourthly, because we did not examine a large number of subjects, we were unable to analyze associations in each allergy disease, namely, pollinosis, allergic asthma, atopic dermatitis, and food allergy. Fifthly, since we did not perform a two-way ANCOVA and multiple regression analysis because of fewer females with current smoking, it is unclear whether our conclusions would be true to females. Finally, since we analyzed the data based on current smoking status, the correlation between the number of cigarettes smoked and IgE is unclear. Therefore, further longitudinal epidemiology studies with a larger sample size of each allergic disease are needed.
Conclusion
In a cross-sectional study of middle-aged and elderly community residents, only among males, the high-IgE group of current smokers had significantly lower intakes of manganese and copper than the low-IgE group. Therefore, it is suggested that IgE levels are more easily increased by allergens in smokers with low manganese and copper intakes. Further large-scale longitudinal studies are needed to prove this causal relationship.
Institutional Review Board Statement
The present study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of Kanazawa University (protocol code, 1491; date of approval, 18 December 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data in the present study are available upon request from the corresponding author. Data are not publicly available due to privacy and ethical policies.
Acknowledgement
The authors thank all the participants in the Shika town study and appreciate the collaborations by all field survey staff and experimental staff.
Conflict of Interest
Author declare no conflict of interest.
References
- Kay AB (2001) Allergy and allergic diseases. First of two parts. N Engl J Med 344(1): 30-37.
- Bantz S, Zhu Z, Zheng T (2014) The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol 5(2): 202.
- Burbank AJ, Sood AK, Kesic MJ, Peden DB, Hernandez ML (2017) Environmental determinants of allergy and asthma in early life. J Allergy Clin Immunol 140(1): 1-12.
- Platts-Mills TAE (2015) The allergy epidemics: 1870–2010. J Allergy Clin Immunol 36(1): 3-13.
- Anyenda EO, Higashi T, Kambayashi Y, Nguyen TTT, Michigami Y, et al. (2016) Associations of cough prevalence with ambient polycyclic aromatic hydrocarbons, nitrogen and sulphur dioxide: a longitudinal study. Int J Environ Res Public Health 13(8): 800.
- Murrison LB, Brandt EB, Myers JB, Hershey GKK (2019) Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest 129(4): 1504-1515.
- Rosa MJ, Jung KH, Perzanowski MS, Kelvin EA, Darling KW, et al. (2011) Prenatal exposure to polycyclic aromatic hydrocarbons, environmental tobacco smoke and asthma. Respir Med 105(6): 869-876.
- Klingbeil EC, Hew KM, Nygaard UC, Nadeau KC (2014) Polycyclic aromatic hydrocarbons, tobacco smoke, and epigenetic remodeling in asthma. Immunol Res 58(2-3): 369-373.
- Qiu F, Liang CL, Liu H, Zeng YQ, Hou S, et al. (2017) Impacts of cigarette smoking on immune responsiveness: Up and down or upside down. Oncotarget 8(1): 268-284.
- Skoner DP (2001) Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol 108(1): S2-8.
- Dong JJ, Shen JJ, Lee YJ (2019) Dose-dependent effect of cotinine-verified tobacco smoking on serum immunoglobulin E Levels in Korean adult males. Nicotine Tob Res 21(6): 813-817.
- Ahmed NJ, Husen AZ, Khoshnaw N, Getta HA, Hussein ZS, et al. (2020) The Effects of smoking on IgE, oxidative stress and haemoglobin concentration. Asian Pac J Cancer Prev 21(4): 1069-1072.
- Bousquet PJ, Cropet C, Klossek JM, Allaf B, Neukirch F, et al. (2009) Effect of smoking on symptoms of allergic rhinitis. Ann Allergy Asthma Immunol 103(3): 195-200.
- Tsuji M, Koriyama C, Ishihara Y, Yamamoto M, Yamamoto-Hanada K, et al. (2019) Associations between metal levels in whole blood and IgE concentrations in pregnant women based on data from the Japan environment and children’s study. J Epidemiol 29(12): 478-486.
- Oluwole O, Arinola OG, Adu MD, Adepoju A, Adedokun BO, et al. (2014) Relationships between plasma micronutrients, serum IgE, and skin test reactivity and asthma among school children in rural southwest Nigeria. J Biomarkers 2014: 1-9.
- Kelley D, Daudu P, Taylor P, Mackey B, Turnlund J (1995) Effects of low-copper diets on human immune response. Am J Clin Nutr 62(2): 412-416.
- Bala S, Failla M, Lunney J (1990) Phenotypic and functional alterations in peripheral blood mononuclear cells of copper‐deficient rats. Ann N Y Acad Sci 587: 283-285.
- Shimizu Y, Kambayashi Y, Tsujiguchi H, Hara A, Hori D, et al. (2018) Relationship between the Use of Parabens and Allergic Diseases in Japanese Adults—A Cross-Sectional Study. J 1(1): 148-158.
- Suzuki F, Morita E, Miyagi S, Tsujiguchi H, Hara A, et al. (2021) Protein intake in inhabitants with regular exercise is associated with sleep quality: Results of the Shika study. PLoS One 16(2): e0247926.
- Yamada Y, Nakamura H, Tsujiguchi H, Hara A, Miyagi S, et al. (2021) Relationships among the β3-adrenargic receptor gene Trp64Arg polymorphism, hypertension, and insulin resistance in a Japanese population. PLoS One 16(8): e0255444.
- Shika Town population (2020).
- Kobayashi S, Murakami K, Sasaki S, Okubo H, Hirota N, et al. (2011) Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr 14(7): 1200-1211.
- Kobayashi S, Honda S, Murakami K, Sasaki S, Okubo H, et al. (2012) Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol 22(2): 151-159.
- Bousquet J, Cabrera P, Berkman N, Buhl R, Holgate S, et al. (2005) The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy 60(3): 302-308.
- Stapleton M, Howard-Thompson A, George C, Hoover RM, Self TH (2011) Smoking and asthma. J Am Board Fam Med 24(3): 313-322.
- Kim SY, Sim S, Choi HG (2017) Active, passive, and electronic cigarette smoking is associated with asthma in adolescents. Sci Reports 7(1): 17789.
- Tanigawa T, Araki S, Nakata A, Sakurai S, Miki A (2010) Effects of smoking and Japanese cedar pollinosis on lymphocyte subpopulations. Arch Environ Health 54(2): 119-123.
- Yoshida T, Usui A, Kusumi T, Inafuku S, Sugiyama T, et al. (2005) A quantitative analysis of cedar pollen-specific immunoglobulins in nasal lavage supported the local production of specific IgE, not of specific IgG. Microbiol Immunol 49(6): 529-534.
- Sakaida H, Masuda S, Takeuchi K (2014) Measurement of Japanese cedar pollen-specific IgE in nasal secretions. Allergol Int 63(3): 467-473.
- Saulyte J, Regueira C, Montes-Martínez A, Khudyakov P, Takkouche B (2014) Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: a systematicreview and meta-analysis. PLoS Med 11(3): e1001611.
- Miah MR, Ijomone OM, Okoh COA, Ijomone OK, Akingbade GT, et al. (2020) The effects of manganese overexposure on brain health. Neurochem Int 135: 104688.
- Wang P, Yuan Y, Xu K, Zhong H, Yang Y, et al. (2021) Biological applications of copper-containing materials. Bioact Mater 6(4): 916-927.
-
Koji Katano, Fumihiko Suzuki, Hiromasa Tsujiguchi, Akinori Hara, Hiroyuki Nakamura etc all. Association of Immunoglobulin E Levels with Smoking and Trace Element Intake: A Cross-sectional Analysis of the Shika Study. Glob J Nutri Food Sci. 3(5): 2022. GJNFS. MS.ID.000571.
-
Cigarette smoking, Serum, Immunoglobulin, Copper, Manganese, Cross-sectional study, Allergic diseases, Logistic regression, Lack of exercise, Sleep deprivation, Current drinker, Bonferroni method
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






