 Review Article
Review Article
The Root of Silica Scale Formation and Its Remedy
Mai Al Saadi* and Naser Al Haddabi
Mechanical Engineering department, Al Musana College of Technology, Oman
Mai Al Saadi, Mechanical Engineering department, Al Musana College of Technology, Oman.
Received Date: September 25, 2019; Published Date: October 03, 2019
Background
Naturally, silica present in water feed in the range of 1-100 ppm. It is existed as silicic acid (H2SiO3) which is a weak acid and dissociated at or below neutral pH [1,2]. The presence of silicic acid lead to form silica colloidal in water at neutral pH. But when pH exceeds the neutral, silicic can be dissociated and form silicate anion (SiO3 2-), which can react with positive ions like calcium, magnesium, iron, manganese and aluminum to form insoluble silicates [3]. It is essential to control the iron content to be minimum as much as possible, at least about 0.05 ppm, in order to avoid corrosion and scale formation. The silica scale formation usually takes place at pH level below 8.5, whereas Magnesium silicate scale form at pH 8.5 and above, because as pH increases the possibility of positive silicates salt to form increases. Silica scale is complex and amorphous product from colloid silica and a complicated mixture of numerous components often including metals ions [3].
Factors of Polymerization Process
Commonly, the concentration of dissolved silica in aquifer waters (200-350 ⁰C) of the geothermal system is strongly high, and it is about 300-700 mg/kg SiO2 (Fournier and Rowe, 1966; Mahon, 1966). Once water contacts the surface, the temperature decreases down to 100 to 200 ⁰C, and the saturation for amorphous silica can occur (Gunnarsson and Arnorsson, 2003). The precipitation of amorphous silica can occur at low temperature below its saturation, otherwise the polymerization will start during saturation point. Uncontrolled precipitation or polymerization can occur over various locations at surface conditions. It is found that silicic acid precipitates on any surface which contain OH groups (Iler, 1979). Hydroxide minerals can grant their OH groups to co-precipitate with amorphous silica. Polymeric silica has lower tendency to precipitate than monomeric silica, because it forms colloids which can remain suspended in the solution. The conditions for polymerization in terms of oversaturation, temperature and pH will be explained. The oversaturation solution can cause polymerization for unionized monomeric silica by reducing the concentration of monomeric silica, for example from 777 mg/kg to 400 mg/kg within first minutes of reaction. Oversaturation of amorphous silica solution is difficult to be treated, and it will lead to polymerization, which increases the risk of silica scaling. Usually, more polymerization can be at low temperature because the concentration of silica will be higher at cooler temperature so that will lead to reduce the concentration of monomeric silica at those temperature. Even though the nature of water is basic and the pH between 8 to 10, the polymerization of monomeric silica has capacity to increase the pH by 0.2 or 0.4, due to removing silicic acid from solution. This can affect the degree of saturation for minerals that have pH-dependent solubility, such as hydroxides, calcite and magnesium silicates [4]. At neutral pH, the precipitation is at the highest amount, while the silica solubility is the lowest (Iler, 1979), in addition, the concentration of monomeric silicic acid polymers is less at pH 11 to 12 dues to strong alkalinity.
Factors of Silica Scale Formation
Silica scale formation usually takes place at pH levels below 8.5, Silica in water can be as reactive silica, colloidal silica and particulate silica. Silicate compounds exist in the form of amorphous, which is soluble in alkali solutions and crystalline form is nonstable. When pH exceed neutral silicic acid can be dissociated and form silicate anions (SiO32-). which can react positive ions like Ca, Mg, Fe, Mn and Al to form insoluble or slight soluble silicate Metal ions such as magnesium and calcium in the water must be taken into account, because at alkaline conditions silica combines with magnesium or calcium ions and forms calcium or magnesium silicate deposits [5]. Magnesium silicate scale forms at a pH above 8.5 in water has high Mg2+ ions. Silicates prefer to react with Magnesium when both Calcium and Magnesium are present. Mg2O4 Si compounds are softer and easier to remove from the equipment surface compared to calcium silicate compounds. Also, it does not adhere to metal surface, nor destroy stainless steel, while the opposite is true with calcium. In addition, Mg concentration can eliminate or minimize calcium scaling in process equipment. At high pH, there is a chance for Mg to react with silicates ions to form silicate compounds. it had found that both Al and Fe ions have high potential to react with silicate so it is crucial to know the source of them and ensure their concentration is less than 0.05 mg/l in feed water. Regarding, aluminum silicates can be soluble at high pH of approximately 7. Therefore, increasing pH will reduce the risk of AL silicate deposition. Low temperature reduces the solubility of amorphous silica (it is any form of silica with lacking in crystal structure such as (Silica gel, Gelatinous gel, Silica sol or Colloidal silica, Opal and Silica glass. However, the solubility of other silicates increases as the temperature increases.
Methods to prevent the occurrence of silica scaling at surface facilities
1. Injection of scaling inhibitor liquid (usually H2SO4) to reduce the pH unit, or caustic soda to rise the pH unit into the brine pipeline in order to stop the formation of scaling for hours (brown, 2011)
2. Separator pressure adjustment can also be applied (sometime unacceptable for economic power generation)
3. Development of double-flash separation leads to higher pH and lower silica scaling.
4. Scale can be avoided by application of three water treatment techniques inhibition of scale deposits, corrosion and prevention of microbiological fouling.
5. Removing silica from the makeup water through “hotlime softening” by precipitation with Mg(OH)2 or MgCl2 followed by filtration, by inhibition (elimination of colloidal silica formation) and by dispersion (stops silica polymerization at early stages by preventing the growth of large particles and their attachment to surfaces) .Example for inhibitors (yellow metal: copper and admiralty brass, polyacrilaies, Boric acid ,dendrimers and quarts), corrosion inhibitors, dispersant polymers and tracers and inhibitors for silica or magnesium silicate are less common. According to one study low dosage of inhibitor can have great influence on Si removal at pH 10.5 and can eliminate 90% of the silicon.
6. Addition of EDTA can inhibit magnesium from catalyzing the polymerization of silicic acid, EDTA is effective and dose dependent when it is high, then it will have a little effect on silica solubility, while low dose provides more effective solubility of silica.
7. The potential of deposition will decrease in the condition of the presence of saline solution, the repulsive forces will reduce which can cause aggregation of silica
Executive method for treating silica scale
Colloidal silica is not affected by the ion exchange process, and when it forms a complex with organic matter it can foul the resins [6]. Colloidal silica is converted into reactive silica at high temperature and pressure, which leads to volatilize them along with steam and get deposited as glassy scale. Some industries have additional investment in order to reduce the number of shutdowns by implementing new technology such as ultra-filtration. There are other means to remove colloidal silica (non- reactive silica). Coagulation which can remove approximately 80 to 90% of colloid silica isn’t easy to implement, because colloids are attached with organic matter which require for pre-chlorination in order to oxidize the organic matter and maintaining optimal pH condition through dosing of a primary coagulant like alum and flocculation as polyelectrolyte [6].
Many industries have addition investment to reduce the risk of silica scale by utilizing ultrafiltration, because it removes the maximum amount of nonreactive silica, firstly by removing the bulk in the pretreatment plant or demineralization process then push it to ultra-filtration (UF) system. It is required be installed at the outlet of the mixed bed (MB unit) and the membranes of UF with molecular weight cut off (MWCO)of 100,000 remove up to 99% while the tighter membrane with an MWCO of 10,000 remove up to 99.8 % [6,7]. For the existing plants which face problems of silica scale, it is good to add hollow fiber UF membranes downstream of the MB unit to capture the colloidal silica that will escape the pretreatment and ion exchange beds (Figure 1).
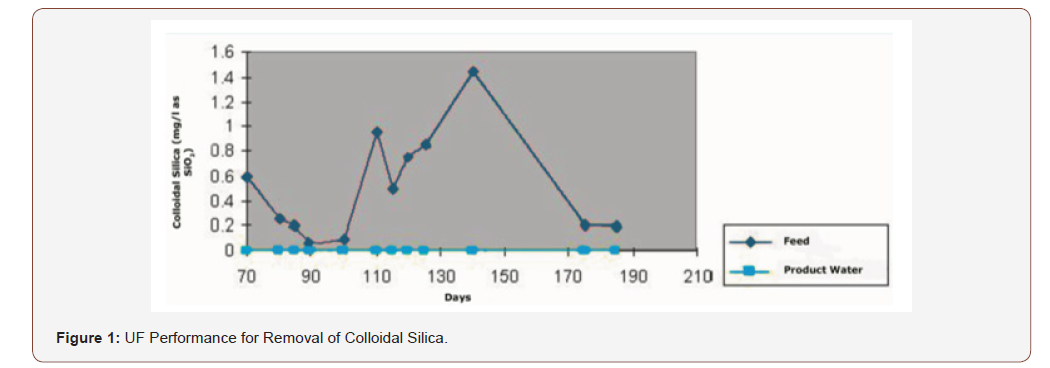
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- (2003) Silica scaling: The main obstacle in efficient use of high-temperature geothermal fluids, in gvi Gunnarsson and Stefan Arnorsson, international Geothermal Conference, Reykjavik, Iceland.
- (2015) Characterization of silica precipitation at Geothermal Condition, Mudhamad, Mariela Hernandez Castaneda, Simona Regenspurg, pproceedings World Geothermal Congress 2015, Melbourne, Australia.
- (2014) The effect of temperature and pH on the formation of silica scaling of Dieng Geothermal field Central Java, Indonesia.
- Winahyu setyo UTAMI, Niniek Rina HERDIANITA, Randy Wijaya ATMAJA, proceedings, thirty Ninth workshop on Geothermal Reservoir Engineering, Standford Unversity, Stanford, California, USA.
- (2004) Scale formation and removal, Kostas demadis, the university of crete Powe, New York 148(6): 19-23.
- (2014) Silica scale inhibition in mechanical pulping, Emine Temiz and Nadieh Sookhtanloo, Chalmers University of technology Goyhenburg, Sweden.
- Colloidal silica Treatment and Removal, water tech 9.
-
Mai Al Saadi, Naser Al Haddabi. The Root of Silica Scale Formation and Its Remedy Glob J Eng Sci. 3(3): 2019. GJES.MS.ID.000565.
-
Numerous components, Amorphous silica, Polymerization, Hydroxides, Ultra filtration, Ultrafiltration, Colloidal silica, Corrosion inhibitors, Admiralty brass, Microbiological fouling
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






