 Research Article
Research Article
Elicitation, A Mechanistic Approach to Change the Metabolic Pathway of Plants to Produce Pharmacological Important Compounds in In-vitro Cell Cultures
Muhammad Naeem Bajwa*1, Amna Bibi2, Muhammad Zaeem Idrees 2, Gohar Zaman2, Umar Farooq1, Talha Tufail Bhatti2
1Department of Biotechnology Quaid-I-Azam University Islamabad
2Department of Chemistry University of Sialkot, Sialkot
Muhammad Naeem Bajwa, Department of Biotechnology Quaid-I-Azam University Islamabad 45320.
Received Date: June 22, 2021; Published Date: July 12, 2021
Abstract
Plant’s secondary metabolites, produced usually under stress, are one of the promising sources for food additives, pharmaceuticals, food flavors and other industrial materials. The comprehensive probing of metabolite’s production mechanism, stress signal transduction pathway, would be great push toward in commercial production. Higher plants inevitably encounter stresses and sustain themselves by producing various secondary metabolites which, the secondary metabolites, have various industrial application that’s why are promising candidates for commercialization. Due to certain limitations of natural plant extraction, plant cell/tissue culture is considered a best alternative way for in-vitro production of bioactive secondary metabolites. Elicitation can be employed to overcome the constraints of plant cell technology that retard the process of commercialization. A way to enhance the secondary metabolite’s production in plants is Elicitation. In which an exogeneous elicitor, biotic or abiotic, is exposed in growth medium to trigger the production of secondary metabolite. During this phenomenon, several defense/ non-defenses related genes, activated/ deactivated. Furthermore, transient phosphorylation or dephosphorylation of proteins, expression of enzymes occurs through which biosynthetic pathways of several secondary metabolites can be ascertained. Additionally, a push toward advancement of metabolic engineering and gene manipulation to increase the productivity of secondary metabolites can be gained through integration of proteomics, transcriptomics, and metabolomics with system biology.
Introduction
Plants are the best reservoir of medicinally important compounds present in their roots, stem, leaves and fruits. These pharmaceutical important phytochemicals produce a protective response against many diseases and illness of humans as well animals [1]. Due to their medicinal uses, they have been used by many cultures throughout the word in traditional herbal remedy against a lot of diseases and illness [2]. Due to rapid development of technologies pharmaceutical industries are showing interest in exploring of such valuable bioactive compounds from plants. Plants possess variety of such pharmaceutically important compounds including flavonoids, terpenoids, saponins and phenolics in various plant parts [2]. These phytochemicals possess specific phytochemical properties against cancer, tumor repression, microbes, and viruses [3]. With the advent of modernization, industrialization, and revolution in medicinal field use of pants and their extracts were reduced due to the availability of synthetic products available for diseases [1]. But due to the hazardous effects of these synthetic drugs trend is changing towards the medicinal plants for treating these dieses. The medicinal plants are safe, cheap and have no side effects on the body as compared to modern synthetic drugs [4]. Reports showed that no. of diseases increased to a large no. due to use of synthetic drugs. Antibiotic resistance is also one of the reasons to go for newer medicines to combat pathogens. several medicinal plants and their pathogens have been used against pathogenic resistant microbes [5].
For large scale production of such highly valuable medicinally important compounds from plants in-vitro culture techniques are used. These invitro culture techniques have advantage over wild grown plants that they have no geographical or seasonal restrictions and have a yield with minimum production time. For large scale industrial production of such compounds in-vitro culture techniques are used in which wild plants are grown under optimal growth conditions and for large biomass production all type of stress to the pants are removed. As a result the secondary metabolite production and therapeutic activities are highly reduced [6].
The plants ‘defense system produces chemicals that cope with the infections caused by internal or external stress. These chemical compounds are the products of secondary metabolism. It means that this process can be enhanced by applying any external stress to increase yield of these compounds, known as secondary metabolites [7]. Various approaches are considered in in-vitro cultures for up gradation or high yield of such vital phytochemicals. One of the most important and efficient strategies is ―Elicitation‖, in which the metabolic pathways are triggered by incorporating agents for optimum production of secondary metabolites. It is an efficient tool usually employed to stimulate phytochemicals yield [8, 9]. For instance, many reports are available on the application of elicitors to employ plants defense mechanisms to enhance the production of these compounds during in-vitro cultures [10]. The elicitors are identified by the cell membrane bounded receptors that activate the signal transduction pathways network by distinct genes, to enhance secondary metabolism [11]. It can also be adopted to characterize and examine the role of different agents on plants by using in-vitro plant cell culture as model system. The agents employed in the process are known as ―Elicitors‖ which generally classified into two; of abiotic or biotic nature [11].
Types of Elicitors
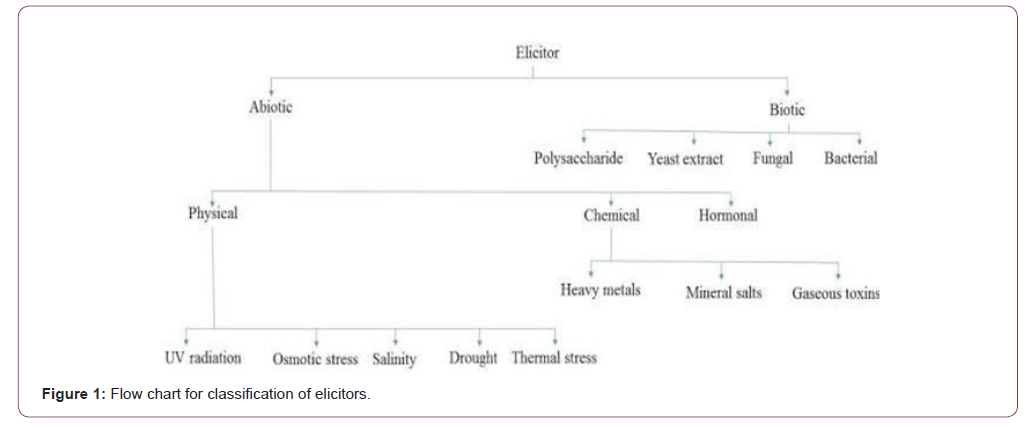
Elicitors can be either physical or chemical in nature. Elicitors can be either biotic or Abiotic according to the nature. The biotic elicitors are such elicitors that are biological nature derived from plants or pathogens while Abiotic elicitors have non biological nature and can be either physical agents or chemicals [7]. In in-vitro plant cell cultures elicitation is the best strategy for the process of fermentation of antibiotics or other fermented products. Elicitation triggers the membrane specific receptors of the metabolic pathway for the enhanced production of secondary metabolites. This strategy can be applied for the large-scale industrial production of commercially viable secondary metabolites. The detailed classification is given in the flow chart Figure 1.
Role of Elicitors in Plant Metabolic Engineering
Biosynthetic pathway for efficient production of secondary metabolites is a challenging issue. Only few metabolic pathways for viable synthesis of secondary metabolites have been discovered so for including flavonoid pathway, terpenoid pathway, indole alkaloid pathway and iso quinoline alkaloid pathways including berberine, morphine production pathways [12-14]. These metabolic pathways have been discovered after an extensive and laborious research work in in-vitro plant cultures. The changing of metabolic pathways in Eukaryotic cells like plants is difficult to attain because these metabolic pathways are complex and triggered by various enzymes which are substrate specific and present in a minute quantity. The changing of metabolic pathway for production of such enzymes is accomplished through metabolic engineering of [15] regulatory genes and their transcription factors [16,17]. So, for most of the studies of plant metabolomics have been carried out mostly to primary metabolites while secondary metabolite production is complex and more challenging due to their highly divergent chemical structures and sensitivities in extraction conditions [15,16].
How elicitors change metabolic pathways
First of all, receptors present on the plasma membrane detect the elicitors and show a signaling response. The effect of elicitors varies from species to species due to their chemical or physical nature [18]. different plant receptors detect the response and show a signal to produce secondary metabolites to minimize the effect of that stress e.g. AVR plant resistance gene products for pathogen avirulent gene [19]. Studies shows that a same elicitor could show response in several plant species it means that different plant species have common receptors for that specific elicitor [20]. The plasma membrane receptors show a signal transduction pathway where various secondary messengers in cell like active oxygen species, free calcium, nitrogen oxide, cGMP, cytosolic PH and cADPR interact in a branched way. As a result, changes in Krebs cycle and Pentose phosphate pathway are signs of serious stress effects on the behaviour of cells and activation of defence responses to minimize that stress [20] phytoalexin and pathogenesis related proteins (PR) are produced in the cell by the activation of the signal transduction pathways [20].
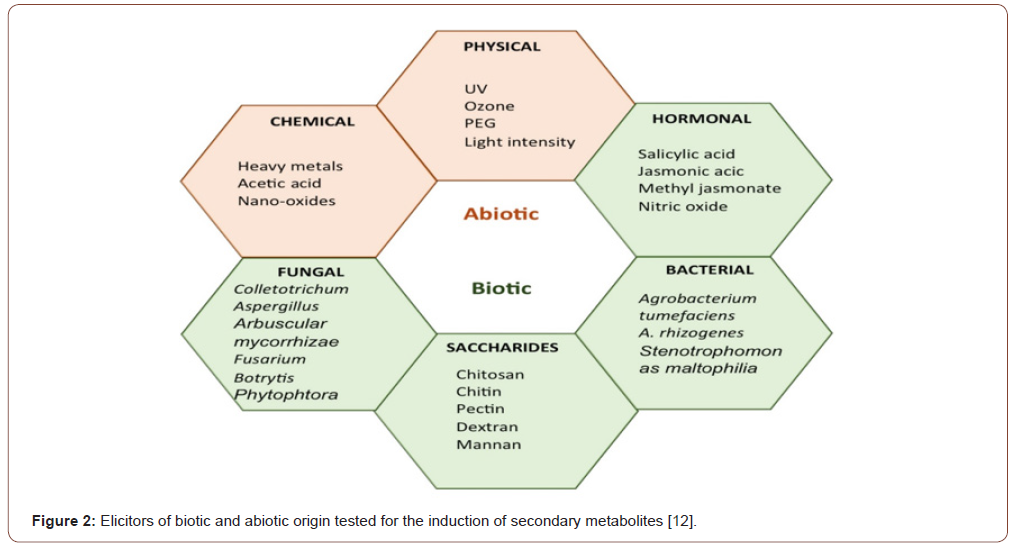
The process of signal transduction is somehow complex and followed by a cascade of reaction where receptors for secondary messenger molecules like active oxygen species, free calcium molecules, nitrogen oxide, PH of cytosol, cADPR and cGMP interact with each other. As a result, changes in Krebs cycle and pentose phosphate pathway occurs that indicates cells behaviour and defence response to minimize that stress [20]. As a result of signal transduction pathways, the plants defence system genes activated that produce some specific compounds like pathogenesis proteins, phytoalexins, and calluses deposition in cell wall to strengthen it [13-21]. These responses are highly specific by various messengers for secondary metabolism and activation and production of target specific proteins [19]. These responses are highly specific and differ from cell to cell and among species [19,24]. Elicitation is mainly used to activate plant defence system as well as production of commercially viable secondary metabolites for cosmetic, food and pharmaceutical industries [25]. According to Giri & Zaheer [26] secondary metabolite production though elicitation of in-vitro cultures can be enhanced up to 1-2230-fold. Moreover, in postharvest treatments, elicitors can increase nutritional value in grapes (enhanced antioxidant properties) [27] or shelf life, in horticultural [28,29] as well as in ginseng roots [30] (Figure 2).
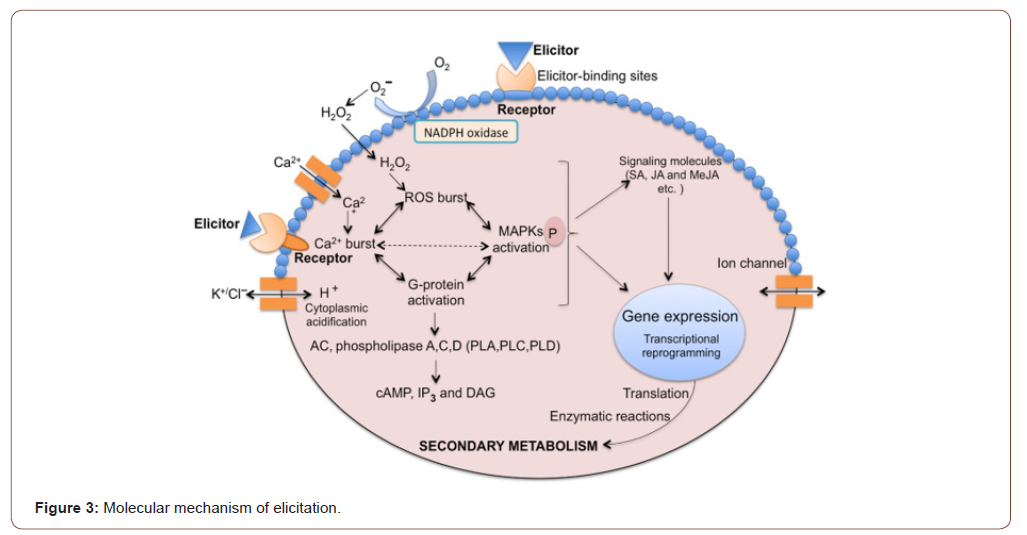
Elicitation is a very complex process carried out by thousands of intertwined events. The mechanism and mode of action of elicitors change with respect to its origin, concentration and specificity nutritional, physiochemical environment, and growth uptake of plants. In the mechanism of elicitation, mitogen-activated protein kinase (MAPK) phosphorylation, reactive oxygen species (ROS) burst, calcium flux are mostly initial events activated in majority of the elicitor to plant cell interactions [31]. Later on, activation of signalling pathways as well as transcription factors that lead to the plant secondary metabolism pathway are being reported [32,33]. The receptors present on plasma membrane recognizes and bind that elicitor and initiates the cascade of events like ion fluxes NADPH oxidase activation , ROS burst, Ca2+ burst, MAPK phosphorylation, cytoplasmic acidification and G-protein activation [10]. Initially the plant responses by the exchange of ions for example K+/Cl− effluxes and Ca2+/H+ influxes. The most important event is Ca2+ influx that involves the physiological processes of the cell [34,35]. This Ca2+ signals produce conformational changes in several Ca2+-binding proteins such as calmodulin like proteins, calcium- dependent kinases (CDPKs) calmodulin and phospholipases as well as by secondary messengers like diacylglycerol (DAG) and inositol 1,4,5- triphosphate IP3 [36]. Ca2+/Calmodulin-mediated pathways show stimulus towards physiological responses of plants and cellular processes like regulation of the oxidative burst, hormonal signalling and gene expression and protein phosphorylation [37]. Another important phenomenon in plant defense system is ROS generation that is produced by oxidases like NADPH oxidase as well as Ca2+ [35, 38]. According to different studies G-proteins play a role in the stimulation of ion channels as well as ROS, phospholipase A, phospholipase C and cell death [36, 39]. The levels of DAG, cAMP, IP3 are stimulated by activated G-proteins that triggers the targeted PKA and PKC. These proteins activate the phosphorylation of MAPKs, that results in transcription, and translation of the gene that leads to enzymatic reactions, which in turn reprogram the pathway of secondary metabolite production.
The Figure 3 shows the molecular mechanism of elicitation: how the plasma membrane- bound receptors recognizes the elicitors that results in ion fluxes, ROS burst, cytoplasmic acidification NADPH oxidase activation, Ca2+ burst,G- protein activation, and mitogen-activated protein kinase phosphorylation. It also activates downstream signalling pathway messengers like salicylic acid, jasmonic acid and methyl jasmonate. Messengers activate transcription factors and gene expression, which lead to reprogramming secondary metabolism [40].
Factors affecting the elicitation process
Elicitation is a complex process that is regulated by several factors [18,25]. the plants produce defence responses to a given elicitor. The concentration of an elicitor play a major role in the production of secondary metabolites [41]. Salicylic Acid (SA) (0,75-5 mM) produced drought tolerance in Eucalyptus globulus and this effect was linked with the concentration of elicitor, showing highest dosage produced tremendous effects. Singh & Usha [42] studied the similar effects in wheat seedlings where water stress conditions were linked to decreased transpiration and improved photosynthesis. Elicitation of chitosan to Basil plant also resulted in lower transpiration that improves plant behaviour under drought conditions [43]. The time of exposure for elicitor is also an important factor for the process of elicitation. The type of cell culture as well as the conditions for the growth room are also the important factors in the process of elicitation [44-47].
Future Perspective
Potential application of nanotechnology
Nano particles are emerging a new class of abiotic elicitors. Studies shows the production of secondary metabolites elicited by different nano particles e.g., the Artemisia annua was elicited for high production of artemisinin through silver oxide nano particles [48]. whereas in in-vitro callus cultures of Calendula officinalis the saponin and carotenoid content was highly increased while anthocyanin and flavonoid contents were decreased by the elicitation of silver nano particles [49]. In in-vitro cultures of Satureja khuzestanica, biosynthesis of secondary metabolites as well as antioxidant capacity was increased by the elicitation of multiwalled carbon nanotubes [50]. Biosynthesis of hypericin and hyperforin production was enhanced through elicitation by zinc and iron nano- oxides nano particles in cell suspension cultures of H. perforatum [51]. Besides the elicitation by metallic nano particles the signaling compounds like SA and MeJA and JA, encapsulated in biodegradable polymers having as that of nano particles for sustained and slow release of signaling molecules for sustainable biosynthesis of secondary metabolites in in-vitro. cell cultures . In cultures of A. Thaliana, metallic nanoparticles elicitation for biosynthesis secondary metabolites were also reported [52,53]. This capacity of secondary metabolites to be adsorbed exhibited linear relation with surface coverage of TiO2 revealing the interrelation of quercetin adsorption with functional surface. By deliberating over this phenomenon, a novel technique, nano trapping strategies for various secondary metabolites, can be established in near future.
A road to drug discovery
Analysis of the new compounds extracted from cells exposed to elicitors can provide us novel drugs and pattern of their bioactivities; bioactivity-guided fractionation can also be employed. Despite the alluring possibility of producing variety of bioactive secondary metabolites(compounds) via tissue cultures and H. perforatum cell through elicitation, commercial implementation of elicitation- based changes in secondary metabolism and pharmacological properties is still in its early stages. To gain substantial amount of aseptic biomass for elicitation is the striking issue currently. In regard to this, small-scale bioreactors of in vitro cultures for obtaining active compounds have been reported [54]. Recently, large scale bioreactor comprising of adventitious root culture for production of H. perforatum phytochemicals have been developed [55,56]. The correct culture vessels selection along with the resolve of exogenous signals required for in vitro production of biomass and optimization of elicitation measures are crucial elements for the further development in this field.
Conclusion
Plants are the reservoir of many useful compounds for nutraceutical, pharmacological and industrial products. Production of such secondary metabolites for industrial scale production in in-vitro cultures is lowered. For production and enhancement of such valuable compounds various techniques are used, elicitation is an effective strategy. The production of secondary metabolites through elicitation varies by the types of cultures, nature and concentration of elicitors, physical conditions of growth chamber and other factors. So, the research is required to optimize the best methods for the optimum production of secondary metabolites. In this study we have discussed how metabolic pathways are changed for secondary metabolite production through changes in genes and production of some specific enzymes.
Acknowledgement
None.
Conflict of Interest
The authors declared no conflict of interest.
References
- Rupela OP, Wani SP, Kranthi, M, Humayun P, Satyanarayana A, et al. (2006) Comparing soil properties of farmers’ fields growing rice by SRI and conventional methods. in Proceedings of 1st National SRI symposium.
- Li FS, JK Weng (2017) Demystifying traditional herbal medicine with modern approach Nature plants 3(8): 1-7.
- Jawdat D (2016) Essential oil profiling in callus of some wild and cultivated Daucus genotypes Industrial Crops and Products 94: 848-855.
- Hussain MI (2011) Eco physiological responses of three native herbs to phytotoxic potential of invasive Acacia melanoxylon R. Br Agroforestry Systems 83(2): 149.
- Fostel JM, PA Lartey (2000) Emerging novel antifungal agents. Drug discovery today 5(1): 25-32.
- Gorelick J, N Bernstein (2014) Elicitation: an underutilized tool in the development of medicinal plants as a source of therapeutic secondary metabolites. Advances in agronomy 124: 201-230.
- Romeo Radman, Teresa Saez, Christopher Bucke, Tajalli Keshavarz (2003) Elicitation of plants and microbial cell systems. Biotechnology and applied biochemistry 37(1): 91-102.
- Yang L, J Stöckigt (2010) Trends for diverse production strategies of plant medicinal alkaloids. Natural product reports 27(10): 1469-1479.
- Yue W, Ming QL, Lin B, Rahman K, Zheng CJ, et al. (2016) Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Critical reviews in biotechnology 36(2): 215-232.
- Zhao J, LC Davis, R Verpoorte (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnology advances23(4):283-333.
- Baenas N, C García Viguera, DA Moreno (2014) Elicitation: a tool for enriching the bioactive composition of foods. Molecules 19(9): 13541-13563.
- Preeti Shakya, Gregory Marslin, Karthik Siram, Ludger Beerhues, Gregory Franklin (2019) Elicitation as a tool to improve the profiles of high‐value secondary metabolites and pharmacological properties of Hypericum perforatum. Journal of Pharmacy and Pharmacology 71(1): 70-82.
- Kucht S (2004) Elimination of ergoline alkaloids following treatment of Ipomoea asarifolia (Convolvulaceae) with fungicides. Planta 219(4): 619-625.
- Winkel Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant physiology126(2): 485-493.
- Grit Rothe, Akira Hachiya, Yasuyuki Yamada, Takashi Hashimoto, Birgit Dräger (2003) Alkaloids in plants and root cultures of Atropa belladonna overexpressing putrescine N‐methyltransferase. Journal of experimental botany 54(390): 2065-2070.
- Oksman Caldentey KM, K Saito (2005) Integrating genomics and metabolomics for engineering plant metabolic pathways Current opinion in biotechnology 16(2):174-179.
- O Fiehn , J Kopka, P Dörmann, T Altmann, RN Trethewey, L Willmitzer (2000) Metabolite profiling for plant functional genomics Nature biotechnology 18(11): 1157-1161.
- Takayuki Tohge, Yasutaka Nishiyama, Masami Yokota Hirai, Mitsuru Yano, Jun-ichiro Nakajima, et al. (2005) Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over‐expressing an MYB transcription factor The Plant Journal 42(2): 218-235.
- Vasconsuelo A, R Boland (2007) Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant science 172(5): 861-875.
- Angela Garcia-Brugger , Olivier Lamotte, Elodie Vandelle, Stéphane Bourque, David Lecourieux, et al.(2006) A Early signaling events induced by elicitors of plant defenses. Molecular plant-microbe interactions 19(7): 711-724.
- Karla Ramirez-Estrada ,Heriberto Vidal-Limon ,Diego Hidalgo ,Elisabeth Moyano ,Marta Golenioswki, et al. (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21(2): 182.
- Sajad Ali, Bashir Ahmad Ganai, Azra N Kamili ,Ajaz Ali Bhat, Zahoor Ahmad Mir (2018) Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiological Research 212: 29-37.
- Thakur M, BS Sohal (2013) Role of elicitors in inducing resistance in plants against pathogen infection: a review. International Scholarly Research Notices.
- Jamioł kowska A (2020) Natural compounds as elicitors of plant resistance against diseases and new biocontrol strategies. Agronomy 10(2): 173.
- Marcela Beatriz Treviño Santa Cruz , Dominique Genoud, Jean-Pierre Métraux, Thierry Genoud (2005) Update in bioinformatics: Toward a digital database of plant cell signalling networks: advantages, limitations and predictive aspects of the digital model. Phytochemistry 66(3): 267-276.
- Narayani M, S Srivastava (2017) Elicitation: a stimulation of stress in in vitro plantcell/tissue cultures for enhancement of secondary metabolite production Phytochemistry reviews 16(6): 1227-1252.
- Giri CC, M Zaheer (2016) Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal Plant Cell, Tissue and Organ Culture (PCTOC) 126(1): 1-18.
- Crupi P (2014) Role of the physical elicitors in enhancing postharvest antioxidant capacity of table grape cv redglobe (Vitis vinifera ) Journal of Food Research 3(2): 61.
- Terry LA, DC Joyce (2004) Elicitors of induced disease resistance in postharvest horticultural crops: a brief review. Postharvest Biology and Technology 32(1): 1-13.
- Feliziani E, L Landi, G Romanazzi (2015) Preharvest treatments with chitosan and other alternatives to conventional fungicides to control postharvest decay of strawberry Carbohydrate polymers 132: 111-117.
- Zhi Liu , Juan Xia , Chong-Zhi Wang , Jin Qiu Zhang , Chang-Chun Ruan , Guang-Zhi Sun , Chun-Su Yuan (2016) Remarkable impact of acidic ginsenosides and organic acids on ginsenoside transformation from fresh ginseng to red ginseng. Journal of agricultural and food chemistry 64(26): 5389-5399.
- Heike Seybold, Fabian Trempel, Stefanie Ranf, Dierk Scheel, Tina Romeis, Justin Lee (2014) Ca2+ signalling in plant immune response: from pattern recognition receptors to Ca2+ decoding mechanisms. New Phytologist 204(4): 782-790.
- Schluttenhofer C, L Yuan (2015) Regulation of specialized metabolism by WRKY transcription factors. Plant Physiology 167(2): 295-306.
- Maeda K (2005) DcMYB1 acts as a transcriptional activator of the carrot phenylalanine ammonia-lyase gene (DcPAL1) in response to elicitor treatment, UV-B irradiation and the dilution effect. Plant molecular biology 59(5): 739752.
- Trewavas AJ, R Malhó (1998) Ca2+ signalling in plant cells: the big network! Current opinion in plant biology 1(5): 428-433.
- White PJ, MR Broadley (2003) Calcium in plants. Annals of botany 92(4): 487-511.
- Meijer HJ, T Munnik (2003) Phospholipid-based signaling in plants. Annual review of plant biology 54(1): 265-306.
- Boudsocq M, J Sheen (2013) CDPK s in immune and stress signaling. Trends in plant science 18(1): 30-40.
- Wojtaszek P (1997) Mechanisms for the generation of reactive oxygen species in plant defence response. Acta Physiologiae Plantarum 19(4): 581-589.
- Roos W, Batsuch Dordschbal, JG Steighardt, Margit Hieke, Dagmar Weiss, Gerhard Saalbach (1999) A redox-dependent, G-protein-coupled phospholipase A of the plasma membrane is involved in the elicitation of alkaloid biosynthesis in Eschscholtzia californica. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1448(3): 390-402.
- Jesus C, Monica Meijon, Pedro Monteiro, Barbara Correia Joana Amaral Mónica Escandon, Maria JesusCanal, et al. (2015) Salicylic acid application modulates physiological and hormonal changes in Eucalyptus globulus under water deficit. Environmental and Experimental Botany 118: 56-66.
- Singh B, K Usha (2015) Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Regulation 39(2): 137-141.
- Malekpoor F, AG Pirbalouti, A Salimi (2016) Effect of foliar application of chitosan on morphological and physiological characteristics of basil under reduced irrigation. Research on Crops 17(2): 354-9.
- Rohwer CL, JE Erwin (2008) Horticultural applications of jasmonates. The Journal of Horticultural Science and Biotechnology 83(3): pp. 283-304.
- Farmer EE, CA Ryan (1990) Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves Proceedings of the National Academy of Sciences 87(19): 7713-7716.
- Saniewski M, K Miyamoto, J Ueda, Methyl jasmonate (1998) induces gums and stimulates anthocyanin accumulation in peach shoots. Journal of Plant Growth Regulation 17(3): 121-124.
- Janoudi A, JA Flore (2003) Effects of multiple applications of methyl jasmonate on fruit ripening, leaf gas exchange and vegetative growth in fruit trees. The Journal of Horticultural Science and Biotechnology 78(6): 793-797.
- Zhang B, Li Ping Zheng, Wan Yi Li, Jian Wen Wang (2013) Stimulation of artemisinin production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Current Nanoscience 9(3): 363-370.
- Ghanati F, S Bakhtiarian (2014), Effect of methyl jasmonate and silver nanoparticles on production of secondary metabolites by Calendula officinalis L (Asteraceae). Tropical Journal of Pharmaceutical Research 13(11): 1783-1789.
- Ghorbanpour M, J Hadian (2015) Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro Carbon 94: 749-759.
- Sharafi E, MH Fotokian, H Loo (2013) Improvement of hypericin and hyperforin production using zinc and iron nano-oxides as elicitors in cell suspension culture of John’swort (Hypericum perforatum L). Journal of Medicinal Plants and By-products 2(2).
- Kurepa J (2014) Direct isolation of flavonoids from plants using ultra‐small anatase TiO2 nanoparticles. The Plant Journal 77(3): 443-453.
- Schlipf DM, Cory A Jones, Marie E Armbruster, Elliott S Rushing, Kaitlyn C Wooten, et al. (2015) Flavonoid adsorption and stability on titania-functionalized silica nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 478: 15-21.
- Zobayed S, S J Murch, H P V Rupasinghe, PK Saxenaa (2004) In vitro production and chemical characterization of St. John’s wort (Hypericum perforatum L. cv ‘New Stem’) Plant Science 166(2): 333-340.
- Wu SQ, Xiao Kun Yu, Mei-Lan Lian, So-Young Park, Xuan-Chun Piao (2014) Several factors affecting hypericin production of Hypericum perforatum during adventitious root culture in airlift bioreactors. Acta physiologiae plantarum 36(4): 975-981.
- Cui XH, HN Murthy, KY Paek (2014) Pilot-scale culture of Hypericum perforatum L. adventitious roots in airlift bioreactors for the production of bioactive compounds. Applied biochemistry and biotechnology 174(2): 784-792.
-
Muhammad Naeem Bajwa, Amna Bibi, Muhammad Zaeem Idrees. Elicitation, A Mechanistic Approach to Change the Metabolic Pathway of Plants to Produce Pharmacological Important Compounds in In-vitro Cell Cultures. Glob J Eng Sci. 8(1): 2021. GJES.MS.ID.000678.
-
Food additives, Pharmaceuticals, Food flavors, Flavonoids, Terpenoids, Saponins, Phenolics, Plasma membrane, Elicitation
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






