 Mini Review
Mini Review
Immunological Microenvironment Associated with the Ovaries and Its Modulation During Aging
Lourdes Materazzi1*, Antonio Cattaneo2*, Lara Castagnola1, Ana Schafir1, Marcela Irigoyen2, Gustavo Martínez2, Diego Gnocchi2, Lautaro Tessari2, Lautaro Fierro2, Esteban Grasso1, María Soledad Gori1 and Rosanna Ramhorst1
1Laboratorio de Inmunofarmacología, Instituto de Química Biológica de la Facultad de Ciencias Exactas y Naturales (IQUIBICEN) CONICET e Universidad de Buenos Aires, Argentina
2FERTILIS Medicina Reproductiva, Buenos Aires, Argentina
Lourdes Materazzi, Laboratorio de Inmunofarmacología, Instituto de Química Biológica de la Facultad de Ciencias Exactas y Naturales (IQUIBICEN) CONICET e Universidad de Buenos Aires, Argentina and Antonio Cattaneo, FERTILIS Medicina Reproductiva, Buenos Aires, Argentina.
Received Date:August 18, 2025; Published Date:August 28, 2025
Abstract
Beyond its roles in gametogenesis and hormone secretion, the ovary encompasses a complex reproductive immunological microenvironment (RIM). This microenvironment is composed of diverse immune cells, including macrophages, T cells, dendritic cells, natural killer (NK) cells, and mast cells. These cells support oogenesis, steroidogenesis, and tissue remodelling. Macrophages stand out among these cells due to their functional plasticity and ability to integrate endocrine, immune, and neural signals. Thus, they participate in virtually all aspects of ovarian physiology. Aging disrupts this finely tuned environment through “inflammaging,” a chronic, low-grade systemic inflammatory state characterized by elevated proinflammatory mediators and tissue fibrosis. In the ovary, inflammaging is associated with structural degeneration, fibro inflammation, and the accumulation of foam cells, which perpetuate inflammation and impair function. Additionally, emerging evidence highlights the role of extracellular vesicles and microRNAs (miRNAs) in regulating intercellular communication within the ovarian niche. This influences both follicular development and the decline in oocyte quality associated with aging. This mini-review discusses the interplay between immune modulation, inflammaging, and microRNA-mediated regulation in the ovarian microenvironment, emphasizing its implications for reproductive aging.
Introduction
Reproductive Aging and the Concept of the Reproductive Immunological Microenvironment (RIM)
Although life expectancy has dramatically increased over the last century, reaching around 85 years for women, the age at menopause has remained relatively constant at about 50 years [1]. Besides their reproductive function, the ovaries play a major role in the secretion of female hormones and, therefore, are involved in various homeostatic processes. With the advent of new technologies that extend people’s lives, we now face an increase in the number of years during which women live in poor health (Figure 1). Thus, reproductive aging is not only relevant from a reproductive standpoint but also for the overall health of women in the second half of their lives.
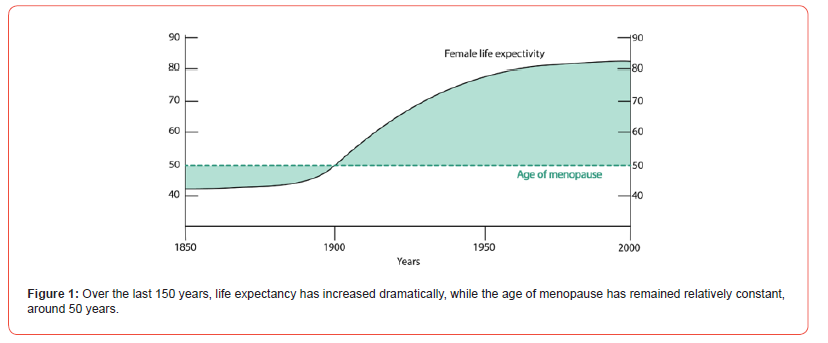
The female reproductive system is one of the first systems to show evident signs of physiological aging. Particularly, the most affected organ is the ovary, which undergoes profound changes that ultimately lead to the cessation of reproductive activity. This process is characterized by a marked reduction in both the quantity and quality of oocytes, a phenomenon known as the maternal age effect [2]. In a typical menstrual cycle, gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the pituitary to release follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which drive ovarian production of estrogens, progesterone, inhibin, and anti-Müllerian hormone (AMH). As women age, follicular depletion reduces these hormones, disrupting feedback to the pituitary and elevating FSH and LH levels. This leads to reproductive aging markers-hormonal changes, irregular cycles, subfertility, infertility, hot flashes, and sleep disturbancesculminating in menopause, which increases risks of depression, osteoporosis, cardiovascular disease, and premature mortality [3].
Reproductive aging is associated with aneuploidy, spontaneous miscarriages, birth defects, and infertility, and these consequences represent a significant social concern, as more and more women worldwide are delaying motherhood due to non-medical reasons [4]. In this regard, ovarian failure due to aging in women of advanced reproductive age is one of the main causes of global infertility (“Female Age-Related Fertility Decline,” 2014) [5], where the success of assisted reproductive techniques is compromised in terms of low fertilization and blastulation rates as well as a high rate of aneuploidies [6], reflected in alarming statistics: up to one in four patients undergoing in vitro fertilization will have a poor reproductive prognosis [7].
Recently, the concept of the Reproductive Immunological Microenvironment (RIM) has emerged as a transformative framework, integrating the immune dynamics of reproductive tissues like the ovarian stroma and endometrium [8]. By offering a macroscopic perspective, the RIM enables a deeper understanding of immune-reproductive interactions. This mini review explores the ovarian immunological microenvironment and its changes during aging, highlighting its implications for reproductive health.
The Ovarian Immunological Microenvironment: Composition and Physiological Functions
The ovary serves dual roles: producing and releasing oocytes for fertilization (gametogenic function) and secreting hormones like estrogens and progesterone to support zygote implantation (endocrine function) [9]. Historically, ovarian research focused on follicle formation, the ovary’s functional unit. However, recent evidence underscores the immune response’s critical role in modulating ovarian function, opening new research frontiers [10]. Structurally, the ovary comprises a cortical zone, housing the ovarian stroma (loose connective tissue, fibroblasts, thecal cell precursors, follicles at various stages, atretic follicles, and corpora lutea), and a medullary zone, rich in vascularization, innervation, and muscle-type cells [11]. In this context, both resident immune cells and those recruited from blood vessels into the follicles can be found.
Immune cells support oogenesis, estrogen synthesis, and antigen presentation [10, 11]. Since Finn (1986) proposed ovulation as an inflammatory process, accumulating evidence has highlighted immune cells’ roles in both generating and regulating this physiological inflammation [12, 11]. Follicular fluid not only represents the microenvironment of the oocyte but is also associated with its quality [13]. Therefore, the characterization of immune cells recovered from the follicular fluid of patients who respond to ovarian stimulation constitutes a highly representative approach to evaluating changes in the ovarian microenvironment. Here we can find immune cells like macrophages, neutrophils, and T lymphocytes, with natural killer (NK) cells and mast cells less common [13, 14, 11].
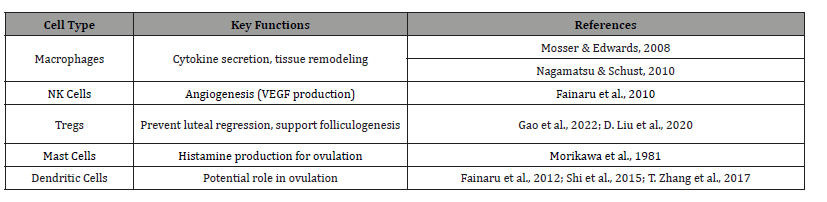
Macrophages, acting as resident “sentinels,” integrate nervous, immune, and endocrine signals, modulating cytokine secretion and ovarian function [15, 16]. Mast cells in the ovarian hilum produce histamine, a mediator of follicle development and ovulation [14]. NK cells, specifically the CD56⁺CD16⁻ subset, promote angiogenesis via vascular endothelial growth factor (VEGF) production [17]. Dendritic cells (DCs) in follicular fluid correlate with gonadotropin response, suggesting a role in ovulation, though their precise functions require further study [18-20]. Regulatory T cells (CD4⁺Foxp3⁺) prevent premature luteal regression and support hormone secretion and follicle development in cases of premature ovarian insufficiency [21, 22]. These immune dynamics, summarized in Table 1, lay the groundwork for understanding how alterations in the ovarian microenvironment contribute to aging-related reproductive decline, as explored in subsequent sections. Given their relevance, we will focus on the contribution of macrophages to ovarian function throughout this work.
Table 1:Key Immune Cells in the Ovarian Microenvironment.
Central Role of Macrophages in Ovarian Function
Macrophages are the predominant immune cells in the ovary, driving essential processes like follicle growth, ovulation, atresia, and corpus luteum formation and regression [23, 24]. Their diverse roles include phagocytosis during atresia and luteolysis, as well as matrix dissolution and tissue remodeling during ovulation and corpus luteum development [25, 26]. A key macrophage function is secreting growth factors and cytokines, such as epidermal growth factor (EGF), insulin-like growth factor (IGF), vascular endothelial growth factor (VEGF), and transforming growth factor (TGF)-α and -β, which regulate primordial follicle development via paracrine signaling. Although granulosa and theca cells also produce these factors, macrophages, as hematopoietic cells, generate them in higher concentrations, underscoring their unique contribution [27].
Macrophages also secrete extracellular vesicles (EVs), nanometer-sized lipid bilayer vesicles that transport proteins, lipids, or RNA for intercellular communication [28]. EV content varies with physiological or pathological contexts, enabling diverse roles in ovarian tissue homeostasis [29]. This plasticity allows macrophages to adopt specialized activated profiles: M1 (proinflammatory, CD11c⁺) and M2 (anti-inflammatory or regulatory, CD206⁺), with murine studies showing M1-like macrophages dominating in inflammatory contexts and M2 macrophages supporting folliculogenesis [30, 31, 15, 32].
Changes in the Immunological Microenvironment During Aging: The Phenomenon of “Inflammaging”
Aging is characterized by structural degeneration, environmental imbalance, functional decline, and reduced adaptability, resilience, and resistance [33]. In recent years, growing evidence has linked inflammation to aging, with the term “inflammaging”-coined by Franceschi et al. in 2000-describing chronic, low-grade inflammation from immune dysregulation [34]. During aging, cells display altered metabolism and mediator expression, secreting pro-inflammatory cytokines, chemokines, and matrix metalloproteinases that facilitate cell-microenvironment communication and immune infiltration [33]. Inflammaging is a hallmark of normal aging and age-related pathologies, including ovarian decline [35]. For instance, Duncan et al. identified an ageassociated cytokine profile in follicular fluid from women aged 27.7-44.8 years, with IL-3, IL-7, IL-15, TGFβ1, TGFβ3, and MIP-1 levels rising with age and inversely correlating with anti-Müllerian hormone [13].
Inflammation also drives fibrosis, where cytokines like IL-1β, IL-6, and TNF-α promote profibrotic effects, leading to tissue and vascular remodelling in aged ovaries [36]. This “fibro inflammation” extends beyond ovaries to general tissue aging and may predict outcomes in assisted reproduction [13]. Aged ovaries exhibit profound changes: low estrogen, follicular depletion, and fibro inflammation. Specifically in ovaries, fibro inflammation accompanies foam macrophages (FMs)-giant cells with lipid-filled cytoplasm [37, 27]. During the luteal phase, phagocytes clear damaged cells for homeostasis, but failure in aged ovaries increases FM frequency and fibrosis [37]. Macrophages show altered metabolism, elevated inflammatory mediators, and fibrosis in aging tissues, including ovaries, where our group and others noted “foamy” macrophages in murine models of advanced reproductive age [37-41].
Foam Macrophages and Their Relevance in Ovarian Aging
Macrophage differentiation into FMs involves LDL influx-efflux dysregulation and PPARγ’s role in metabolism, inflammation, and cholesterol efflux via LDL receptors and ABCA1 [42]. Chronic phagocytosis of apoptotic bodies or lipids in inflammatory contexts leads to non-digestible intracellular accumulation, forming FMs [43]. Lipid-laden FMs secrete pro-inflammatory cytokines (IL- 1α, IL-6, TNF-α), creating a feedback loop that sustains chronic inflammation. Efferocytosis, essential for homeostasis, becomes pathological when excessive. Anti-inflammatory therapies, such as EV injections from anti-inflammatory macrophages in aged mice, restore ovarian function, fertility, and oocyte quality while reducing cytokines [44]. Murine studies distinguish resident ovarian macrophages (self-proliferating) from blood-recruited monocytes [45]. Aging depletes residents, replacing them with monocyte-derived M1 macrophages that amplify inflammation [46, 47]. Future research should explore macrophage population shifts, functions, and metabolism to elucidate their role in ovarian aging.
Modulation by miRNAs in Follicular Development and Ovarian Aging
Folliculogenesis, encompassing reactivation, selection, growth, atresia, and ovulation, relies on precise gene regulation by endocrine and paracrine factors [48]. MicroRNAs (miRNAs), small (~22-nucleotides), non-coding RNAs, regulate mRNA translation by targeting messenger RNAs via sequence complementarity, modulating gene expression critical for follicular development [49].
Essential for development, differentiation, and homeostasis, miRNA biogenesis machinery disruptions are lethal in mouse embryos [49, 50]. Beyond intracellular roles, miRNAs are secreted in stable forms within small extracellular vesicles (sEVs; 30-100 nm) and microvesicles (MVs; 100 nm-1 μm) in body fluids like serum and follicular fluid (FF), acting as hormone-like signaling molecules [51]. These extracellular miRNAs facilitate intercellular communication, influencing gene expression under physiological and pathological conditions [52]. Notably, macrophages-as predominant immune cells in the ovary-secrete EVs containing miRNAs, which could exert paracrine effects on nearby cells, including other macrophages, potentially modulating inflammatory responses during ovarian aging. In this regard, both in vitro and in vivo studies have revealed that these vesicles can be taken up by granulosa cells, suggesting a role in intercellular communication [52].
Most primordial follicles remain in a dormant state, while a small number become activated and recruited into the growing pool. Among these growing follicles, only one will be selected as the dominant follicle, while the rest degenerate and become atretic. Specific families and clusters of miRNAs have been identified as being involved in dominant follicle development, such as miR- 21, the let-7 family, and the miR-17-92 cluster [53-55]. Likewise, together with other factors and hormones, miRNAs appear to play a crucial role during the atresia process. Some of the most wellcharacterized miRNAs include the let-7 family, miR-22, and the miR-23-27-24, miR-183-96-182, and miR-17-92c clusters [55]. In an in vitro study, overexpression of miR-23 and miR-27 in human granulosa cells was found to promote apoptosis via the FAS-FASL pathway, through the regulation of their target gene SMAD5 [50].
FF, derived from plasma and granulosa/theca cell secretions, shapes oocyte development [56]. miRNAs in FF EVs mirror those in granulosa and cumulus cells, suggesting their potential as biomarkers for ovarian function [57]. In women with reduced ovarian reserve or advanced maternal age, FF miRNA profiles (e.g., hsa-miR-21-5p, hsa-miR-134, hsa-miR-190b, hsa-miR-99b-3p) differ, correlating with aging and oocyte quality [58, 59]. These findings confirm previous studies on the role of advanced maternal age in shaping the composition of follicular fluid microenvironments and provide further evidence supporting the use of extracellular miRNAs in FF as potential biomarkers for assessing oocyte quality.
Moreover, various research groups have reported the effects of different miRNAs on granulosa cell function and survival, identifying altered miRNAs in women with premature ovarian failure, both at early stages and in its fully manifested form [60, 61]. Among others, it is noteworthy that overexpression of miR-133b inhibits estradiol production by granulosa cells, whereas overexpression of miR-3061-5p inhibits their proliferation-both leading to premature ovarian failure [62, 63]. These miRNA-mediated changes may interact with the ovarian immunological microenvironment, potentially modulating macrophage activity during inflammaging, as explored later.
Conclusion and Future Perspectives
The ovary is not only a heterogeneous organ in terms of the cell populations it contains, but also in terms of time, throughout a woman’s life. Until now, the mechanism of ovarian reserve decline has not been fully understood. However, experimental evidence to date indicates that ovarian aging is accompanied by an increase in the sustained fibroinflammatory response over time and a decrease in oocyte quality.
Some of the evidence includes: (1) Transcriptomic analyses show an increase in the expression of genes associated with the inflammatory response in the ovaries and follicles recovered from women of advanced reproductive age compared to younger women (Duncan et al., 2017; [47]; (2) Ovaries of advanced reproductive age exhibit excessive collagen deposits in the ovarian stroma that are consistent with tissue fibrosis [37]; (3) Histological sections of ovaries from aged mice revealed the presence of foamy macrophages, which may be associated with chronic inflammatory processes [37]. Further studies are still needed to investigate the impact of the immune response on ovarian stromal cell function and follicular development in aged ovaries. Knowledge of the generation of chronic inflammation associated with aging, as well as other ovarian pathologies such as polycystic ovary syndrome and premature ovarian failure, is advancing in a new immunomodulatory therapeutic approach.
Regarding miRNAs, they regulate various biological processes in the ovary, including folliculogenesis, ovulation, and hormone production. Their association with reproductive disorders, such as polycystic ovary syndrome and premature ovarian failure, highlights the importance of miRNAs as modulators of ovarian homeostasis and as mediators of pathological alterations. Furthermore, the identification of specific miRNA profiles associated with these conditions offers a promising opportunity for their use as biomarkers, not only for diagnosis but also for the early detection of ovarian pathologies. Future research in this field will allow a better understanding of their function and the exploitation of their clinical potential in the management of reproductive disorders. Integration of inflammatory signatures with miRNA profiling may provide a powerful tool to confront the reproductive impact of delayed childbearing.
Acknowledgement
None.
Conflict of interest
No conflict of interest.
References
- Zhu Z, Xu W, Liu L (2023) Ovarian aging: mechanisms and intervention strategies. Medical Review 2(6): 590-610.
- Broekmans FJ, Soules MR, Fauser BC (2009) Ovarian Aging: Mechanisms and Clinical Consequences. Endocrine Reviews 30(5): 465-493.
- Inman Z, Flaws JA (2024) Endocrine Disrupting Chemicals, Reproductive Aging, and Menopause: A Review. Reproduction 168(5): e240113
- Molina-García L, Hidalgo-Ruiz M, Cocera-Ruíz EM, Conde-Puertas E, Delgado-Rodríguez M, et al. (2019) The delay of motherhood: Reasons, determinants, time used to achieve pregnancy, and maternal anxiety level. PLOS ONE 14(12): e0227063.
- Female age-related fertility decline. (2014). Fertility and Sterility 101(3): 633-634.
- Kasapoğlu I, Seli E (2020) Mitochondrial Dysfunction and Ovarian Aging. Endocrinology 161(2): bqaa001.
- Galatis D, Kalopita K, Grypiotis I, Flessas I, Kiriakopoulos N, et al. (2022) Researching the Phenomenon of Poor Ovarian Responders and Management Strategies in IVF: A Narrative Review. Acta Medica Academica 51(2): 108-122.
- Lv H, Zhao G, Jiang P, Wang H, Wang Z, et al. (2022) Deciphering the endometrial niche of human thin endometrium at single-cell resolution. Proceedings of the National Academy of Sciences: 119(8): e2115912119.
- Yang F, Zheng Q, Jin L (2019) Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Frontiers in Immunology: 10: 2317.
- Kinnear HM, Tomaszewski CE, Chang AL, Moravek MB, Xu M, et al. (2020) The ovarian stroma as a new frontier. Reproduction 160(3): R25-R39.
- Yang X, Gilman-Sachs A, Kwak-Kim J (2019) Ovarian and endometrial immunity during the ovarian cycle. Journal of Reproductive Immunology 133: 7-14.
- Finn C A (1986) Implantation, Menstruation and Inflammation. Biological Reviews 61(4): 313-328.
- Machlin JH, Barishansky SJ, Kelsh J, Larmore MJ, Johnson BW, et al. (2021) Fibroinflammatory Signatures Increase with Age in the Human Ovary and Follicular Fluid. International Journal of Molecular Sciences 22(9): 4902.
- Morikawa H, Okamura H, Takenaka A, Morimoto K, Nishimura T (1981) Histamine concentration and its effect on ovarian contractility in humans. International Journal of Fertility 26(4): 283-286.
- Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nature Reviews Immunology 8(12): 958-969.
- Nagamatsu T, Schust DJ (2010) The Immunomodulatory Roles of Macrophages at the Maternal-Fetal Interface. Reproductive Sciences 17(3): 209-218.
- Fainaru O, Amsalem H, Bentov Y, Esfandiari N, Casper RF (2010) CD56brightCD16- natural killer cells accumulate in the ovarian follicular fluid of patients undergoing in vitro fertilization. Fertility and Sterility 94(5): 1918-1921.
- Fainaru O, Hantisteanu S, Rotfarb N, Michaeli M, Hallak M, et al. (2012) CD11c+HLADR+ dendritic cells are present in human ovarian follicular fluid, and their maturity correlates with serum estradiol levels in response to gonadotropins. Fertility and Sterility 97(3): 702-706.
- Shi SL, Peng ZF, Yao GD, Jin HX, Song WY, et al. (2015) Expression of CD11c+HLA-DR+dendritic cells and related cytokines in the follicular fluid might be related to pathogenesis of ovarian hyperstimulation syndrome. International Journal of Clinical and Experimental Pathology 8(11): 15133-15137.
- Zhang T, Tian F, Huo R, Tang A, Zeng Y, et al. (2017) Detection of dendritic cells and related cytokines in follicular fluid of patients with polycystic ovary syndrome. American Journal of Reproductive Immunology: 78(3).
- Gao H, Gao L, Wang W (2022) Advances in the cellular immunological pathogenesis and related treatment of primary ovarian insufficiency. American Journal of Reproductive Immunology 88(5): e13622.
- Liu D, Tu X, Huang C, Yuan Y, Wang Y, et al. (2020) Adoptive transfers of CD4+ CD25+ Tregs partially alleviate mouse premature ovarian insufficiency. Molecular Reproduction and Development 87(8): 887-898.
- Tingen CM, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, et al. (2011) A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. REPRODUCTION 141(6): 809-820.
- Turner EC, Hughes J, Wilson H, Clay M, Mylonas KJ, et al. (2011) Conditional ablation of macrophages disrupts ovarian vasculature. REPRODUCTION 141(6): 821-831.
- Rodgers RJ, Lavranos TC, van Wezel IL, Irving-Rodgers HF (1999) Development of the ovarian follicular epithelium. Molecular and Cellular Endocrinology 151(1-2: 171-179.
- Rodgers RJ, Irving-Rodgers HF (2010) Formation of the Ovarian Follicular Antrum and Follicular Fluid. Biology of Reproduction 82(6): 1021-1029.
- Foley KG, Pritchard MT, Duncan FE (2021) Macrophage-derived multinucleated giant cells: hallmarks of the aging ovary. Reproduction 161(2): V5-V9.
- Machtinger R, Laurent LC, Baccarelli AA (2016) Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Human Reproduction Update 22(2): 182-193.
- Paul N, Sultana Z, Fisher JJ, Maiti K, Smith R (2023) Extracellular vesicles- crucial players in human pregnancy. Placenta 140: 30-38.
- Erlebacher A (2013) Immunology of the Maternal-Fetal Interface. Annual Review of Immunology 31(1): 387-411.
- Hesketh M, Sahin KB, West ZE, Murray RZ (2017) Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. International Journal of Molecular Sciences 18(7): 1545.
- Pepe G, Locati M, Della Torre S, Mornata F, Cignarella A, et al. (2018) The estrogen-macrophage interplay in the homeostasis of the female reproductive tract. Human Reproduction Update 24(6): 652-672.
- Huang Y, Hu C, Ye H, Luo R, Fu X, et al. (2019) Inflamm-Aging: A New Mechanism Affecting Premature Ovarian Insufficiency. Journal of Immunology Research: 8069898.
- Li X, Li C, Zhang W, Wang Y, Qian P, et al. (2023) Inflammation and aging: signaling pathways and intervention therapies. Signal Transduction and Targeted Therapy 8(1): 239.
- Chung HY, Kim DH, Lee EK, Chung KW, Chung S, et al. (2019) Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging and Disease 10(2): 367-382.
- Zhou Y, Ding X, Wei H (2022) Reproductive immune microenvironment. Journal of Reproductive Immunology 152: 103654.
- Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, et al. (2016) Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction 152 (3): 245-260.
- Castagnola L, Gallino L, Schafir A, Vota D, Grasso E, et al. (2025) Ovarian premature aging: VIP as key regulator of fibro-inflammation and foamy macrophages generation. Molecular and Cellular Endocrinology 599: 112486.
- Lujambio A (2016) To clear, or not to clear (senescent cells)? That is the question. BioEssays: 38(S1) P: S56-S64.
- Malaquin N, Martinez A, Rodier F (2016) Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype. Experimental Gerontology 82: 39-49.
- Rao SG, Jackson JG (2016) SASP: Tumor Suppressor or Promoter? Yes! Trends in Cancer 2(11): 676-687.
- Watson MG, Chambers KL, Myerscough MR (2023) A Lipid-Structured Model of Atherosclerotic Plaque Macrophages with Lipid-Dependent Kinetics. Bulletin of Mathematical Biology 85(9): 85.
- Ford HZ, Zeboudj L, Purvis GSD, Ten Bokum A, Zarebski AE, et al. (2019) Efferocytosis perpetuates substance accumulation inside macrophage populations. Proceedings of the Royal Society B: Biological Sciences 286(1904): 20190730.
- Xiao Y, Peng X, Peng Y, Zhang C, Liu W, et al. (2022) Macrophage‐derived extracellular vesicles regulate follicular activation and improve ovarian function in old mice by modulating local environment. Clinical and Translational Medicine: 12(10):
- Ginhoux F, Guilliams M (2016) Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 44(3): 439-449.
- Zhang Z, Schlamp F, Huang L, Clark H, Brayboy L (2020) Inflammaging is associated with shifted macrophage ontogeny and polarization in the aging mouse ovary. Reproduction 159(3): 325-337.
- Zhang Z, Huang L, Brayboy L (2021) Macrophages: an indispensable piece of ovarian health. Biology of Reproduction 104(3): 527-538.
- Chen Y, Wang S, Zhang C (2025) The Differentiation Fate of Granulosa Cells and the Regulatory Mechanism in Ovary. Reproductive Sciences 32(5): 1414-1426.
- Chong MMW, Zhang G, Cheloufi S, Neubert TA, Hannon GJ, et al. (2010) Canonical and alternate functions of the microRNA biogenesis machinery. Genes & Development 24(17): 1951-1960.
- Nie M, Yu S, Peng S, Fang Y, Wang H, et al. (2015) miR-23a and miR-27a Promote Human Granulosa Cell Apoptosis by Targeting SMAD51. Biology of Reproduction: 93(4): 98.
- Wang K, Zhang S, Weber J, Baxter D, Galas DJ (2010) Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Research 38(20): 7248-7259.
- Xie J, Xu X, Liu S (2023) Intercellular communication in the cumulus–oocyte complex during folliculogenesis: A review. Frontiers in Cell and Developmental Biology 11: 1087612.
- Bahmyari S, Jamali Z, Khatami SH, Vakili O, Roozitalab M, et al. (2021) microRNAs in female infertility: An overview. Cell Biochemistry and Function 39(8): 955-969.
- Naji M, Aleyasin A, Nekoonam S, Arefian E, Mahdian R, et al. (2017) Differential Expression of miR-93 and miR-21 in Granulosa Cells and Follicular Fluid of Polycystic Ovary Syndrome Associating with Different Phenotypes. Scientific Reports 7(1): 14671.
- Zhang J, Xu Y, Liu H, Pan Z (2019) MicroRNAs in ovarian follicular atresia and granulosa cell apoptosis. Reproductive Biology and Endocrinology 17(1): 9.
- Revelli A, Piane LD, Casano S, Molinari E, Massobrio M, et al. (2009) Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reproductive Biology and Endocrinology 7(1): 40.
- da Silveira JC, de Ávila ACFCM, Garrett HL, Bruemmer JE, Winger QA, et al. (2018) Cell-secreted vesicles containing microRNAs as regulators of gamete maturation. Journal of Endocrinology 236(1): R15-R27.
- Diez-Fraile A, Lammens T, Tilleman K, Witkowski W, Verhasselt B, et al. (2014) Age-associated differential microRNA levels in human follicular fluid reveal pathways potentially determining fertility and success of in vitro Human Fertility 17(2): 90-98.
- Zhao H, Wang L, Wang Y (2021) Circulating microRNAs as candidate biomarkers for the ovarian response during in vitro fertilization. Medicine 100(6): e24612.
- Liu B, Liu L, Sulaiman Z, Wang C, Wang L, et al. (2024) Comprehensive analysis of lncRNA-miRNA-mRNA ceRNA network and key genes in granulosa cells of patients with biochemical primary ovarian insufficiency. Journal of Assisted Reproduction and Genetics 41(1): 15-29.
- Xiao S, Du J, Yuan G, Luo X, Song L (2024) Granulosa Cells-Related MicroRNAs in Ovarian Diseases: Mechanism, Facts and Perspectives. Reproductive Sciences 31(12): 3635-3650.
- Dai A, Sun H, Fang T, Zhang Q, Wu S, et al. (2013) MicroRNA‐133b stimulates ovarian estradiol synthesis by targeting Foxl2. FEBS Letters 587(15): 2474-2482.
- Liu T, Wen Y, Cui Z, Chen H, Lin J, et al. (2024) MicroRNA ‐3061 downregulates the expression of PAX7/Wnt/Ca signalling axis genes to induce premature ovarian failure in mice. Cell Proliferation: 57(11): e13686.






