 Case Report
Case Report
Vascular Injuries During A Robotic Left Radical Nephrectomy: Case Report
Mahmoud I Khalil, Benjamin Schurhamer and Mohamed H Kamel*
Department of Urology, University of Arkansans for Medical Sciences, Little Rock, Arkansas, USA
Dr. Mohamed Kamel, Department of Urology, University of Arkansans for Medical Sciences, Little Rock, Arkansas, USA.
Received Date: September 06, 2019; Published Date: September 20, 2019
Abstract
We describe a case of 57year-old Caucasian female who presented with a 9cm mostly endophytic solid left renal mass to the urologic-oncology service at our institution. The patient elected for a robot-assisted laparoscopic left radical nephrectomy. During the procedure, we encountered inadvertent injuries to both the contralateral right renal artery and the superior mesenteric artery (SMA). We promptly identified the injuries and we immediately summoned the vascular surgery team and converted to open surgery. Vascular surgery team performed an end to end repair to the injured right renal artery and placed a polytetrafluoroethylene (PTFE) graft to repair the injured SMA. The kidney showed signs of recovery in the immediate postoperative period and the bowel looked viable as well. However, eventually the small bowel suffered massive gangrene and sadly, the patient died on postoperative day 4.
Keywords: Robotic; Nephrectomy; Superior mesenteric artery; Renal artery; Contralateral; Injury
Abbreviations: OR: Operating Room; PTFE: Polytetrafluorethylene; RP: Radical Prostatectomy; SMA: Superior Mesenteric Artery.
Case Report
A 57-year female patient presented with a solid left renal mass to the urologic oncology clinic (Figure 1). The renal mass was an incidental finding during investigation for lower back pain. Otherwise, the patient was relatively healthy. We counseled the patient on her treatment options including open versus minimally invasive radical nephrectomy. The patient felt appealed to undergo a robotic left radical nephrectomy. The counselling also involved the potential complications of left radical nephrectomy (whether open/robotic) including injuries to the superior mesenteric artery (SMA), and the potential fatal outcome of such an injury as well as any other injuries involving the Aorta.

The surgery performed in an academic institution with an active accredited urology residency program. At our institution, we strictly enforce the steps recommended by the Joint Commission to prevent wrong site surgery. This included appropriate preoperative history taking, appropriate side and procedure planned on the consent form, the use of a sufficiently permanent unambiguous mark on the correct side of the surgery, a thorough timeout with the attending surgeon present in the room and display of patient imaging at the time of the surgery [1].
The surgery started with the resident at the robotic console and the resident assigned the initial part of the procedure; which is the division of the white line of Toldt and medial mobilization of the descending colon. The resident has performed this part of the procedure in different radical nephrectomies, in addition to regular training to our residents in dry/animal labs on laparoscopic radical nephrectomy procedure. The attending surgeon was at the bedside assisting and keeping a very close eye on the resident and doing his best to give him instructions to stay in the correct planes. However, he struggled to progress and consequently the surgeon switched position with the resident, and it is felt that the initial dissection was carried in the wrong plane. As the surgeon was getting into the correct plane, two arteries, clearly coursing in the lateral direction, in the direction of the left kidney encountered. The left kidney is more likely than the right kidney to have aberrant/accessory vessels, which can originate directly from the aorta or nearby arteries; of note one of them was small in caliber. The surgeon checked that the small bowel remained healthy, pink, with good peristalsis all the time, and after controlling these two vessels. During the procedure of radical nephrectomy, we usually control the renal artery before the vein. However, after controlling the left renal vein at the hilar area with the stapler, another artery that seemed to be the true left renal artery was found. At that point, there was a concern about the prior secured arteries. The surgeon continued his due diligence, and upon further dissection, identified an injury to the SMA and the right renal artery. At that point, he immediately summoned the vascular surgery team into the operating room (OR). The robot was undocked, and the case was converted into open surgery to repair the vascular injuries.
The vascular surgery team arrived within 10-15 minutes and during that time; the OR team was setting the field ready to avoid any delays. A bilateral Chevron incision was performed, and vascular surgery team explored the major vessels. They elected to start with the repair of the right renal artery to minimize the warm ischemia time. 10,000 units of heparin intravenously was injected. The repair of the right renal artery was carried out through an endend anastomosis, and with adequate perfusion to the right kidney. Then they turned their attention to repair the injured SMA using a polytetrafluoroethylene (PTFE) graft interposed between the severed ends of the SMA. A Doppler check on the repaired arteries showed good blood flow in both. At the end of the procedure, the bowel was pink and healthy, and the right kidney was producing urine while the patient was still on the OR table. The surgical team elected not to close the fascia to allow for a second inspection of the bowel and the right kidney the following day, and consequently, only closed the skin.
Postoperatively, the patient was admitted to the intensive care unit, and overnight she did well and was hemodynamically stable. The total urine output measured the following day was > 2 L. The following day, surgery team took the patient back to the OR and a thorough bowel inspection revealed the bowel was healthy and the kidney was well perfused. However, the gallbladder was gangrenous and consequently a cholecystectomy was performed, and a pack was left in the renal bedside since there was a hematoma. They elected at that time to continue not to close the fascia and to bring the patient for a second inspection of the bowel the following day and for pack removal. During the second inspection, the bowel was found healthy except for a small portion of the bowel less than an inch width that looked gangrenous and performed resection of that portion of the bowl with end-to-end anastomosis using intestinal stapling device, they removed the pack, and closed the fascia.
We transferred the patient back to the intensive care unit. However, over the subsequent 48 hours, the patient continued to deteriorate, with increased output from the nasogastric tube, rising lactate levels, and started on hemodialysis. On postoperative day 4, an x-ray of the abdomen showed free air in her abdomen and subsequent computerized tomography scan performed the same day showed pneumatosis intestinalis, and a patent right renal artery with a well-perfused right kidney. The surgery team took the patient back to the OR and exploration showed complete small bowel gangrene, an unlivable condition and after consulting with the family the decision was not to pursue any further treatments. Sadly, the patient died few hours after this exploration. The main findings of the postmortem exam showed a patent PTFE graft, gangrenous small bowel and grossly normal looking right kidney with patent right renal artery.
Discussion
We described the case of known vascular injuries that can happen during a left radical nephrectomy. The surgeon did not miss these injuries and despite promptly seeking appropriate help from an experienced vascular surgery team, we encountered a fatal outcome. Academic institutions across the United States carry the burden of treating future surgeons. In the surgical specialty of urology, robotic training to the residents is a requirement by the Accredited Committee for Graduate Medical Education (ACGME) [2]. In an effort to provide optimal training, virtual-reality robotic simulators and porcine models have been increasingly involved in urology residency training programs [3]. Of the training protocols described, initial utilization of simulation followed by bedside assisting and finally participating in the robotic console [4, 5]. Schroeck FR, et al. [6] demonstrated that resident involvement in robotic prostatectomy (RP) did not affect outcomes, including operative time, blood loss, and positive surgical margin rates [6]. Similarly, Schommer E, et al. [7] reported that supervised resident involvement in RP did not negatively affect perioperative patient outcomes, although, it prolongs operating time compared to surgeon-only cases [7].
Regarding the anatomy of the renal vasculature and the surrounding structures, there are important anatomical points. The renal arteries are the only vascular supply to the kidneys. They arise from the lateral aspect of the abdominal aorta, typically at the level of the L1/L2 intervertebral disk, immediately inferior to the origin of the SMA. They enter the renal hilum anterior to the renal pelvis and posterior to the renal vein. The right renal artery originates from the anterolateral aspect of the aorta and runs posterior to the inferior vena cava to reach the right kidney, while the left renal artery originates slightly higher and from a more lateral aspect of the aorta, and runs almost horizontally to the left kidney [8-10]. Consequently, the origins of both the right and left renal arteries lie beneath the left renal vein. This may have contributed to the right renal artery injury that we encountered and the reason there are reported several incidences on contralateral renal artery injury during a left radical nephrectomy [11].
Ozkan U, et al. [12] studied the origins and variations of renal arteries among 855 consecutive patients using non-selective catheterization during aortofemoropopliteal (AFP) angiography and by selective or non-selective catheterization during renal angiography. Authors found that in 98% of patients the main renal arteries origin was between the upper margin of L1 and lower margin of L2 vertebra with the L1-L2 intervertebral disc being the most common location. Extra-renal arteries (24%) were located on the right and left side in 16% and 13% of cases, respectively. Of all the extra-renal arteries, the percentage of aberrant renal arteries was marginally higher than accessory renal arteries (51% vs 49%, respectively) [12]. Of note, aberrant renal arteries perforate the substance of the kidney rather than entering its hilum and could arise as high as inferior phrenic artery or as low as internal iliac arteries. Also, Bordei P, et al. [13] presented 54 cases of double renal arteries supplying one kidney and originating from the aorta. They found that of the 54 cases, 42 were unilateral, showing a left predominance (25 cases, 59.5%), three of them with triple renal arteries on the opposite side [13].
Understanding the impact of cutting the blood supply on the kidney and its effect on nephron function comes best from studies reporting on the renal functions of partial nephrectomy. It is well known that during the procedure of partial nephrectomy, the urologist would clamp the renal artery and for a prolonged period in order to excise the tumor and repair the defect and with near complete recovery of kidney function. Lee et al. studied the renal functions outcome of 1816 kidneys underwent partial nephrectomy. The authors concluded that a prolonged warm ischemia time (≥ 50 minutes) was not associated with increased risk of chronic kidney disease [as defined as an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73m2] and major renal function deterioration (defined as an estimated GFR decrease of ≥ 25% postoperatively). The authors compared this group of patients to those underwent partial nephrectomy and with shorter warm ischemia time (< 30 minutes) [14]. Mir MC, et al. [15] conducted a thorough literature review on the impact of warm ischemia on the kidney following partial nephrectomy and concluded the use of a single cutoff for duration of ischemia time as a dichotomous value for renal function outcomes in the setting of partial nephrectomy (commonly 30 minutes) is flawed. The authors added that renal ischemia is a controversial topic and likely, the kidney can tolerate ischemia times of more than 30 minutes without a clinically significant decline in renal function [15]. More recently, there is a shift in the thought process of understanding the renal functions following a partial nephrectomy with more emphasis on the volume of the excised renal mass as the primary role in determining the renal functions outcomes and with the warm ischemia time playing secondary roles [16]. In our case, the right renal unit was intact. In addition, animal models have shown that the kidney can withstand warm ischemia times of up to 90 minutes [17]. The literature reveals it might take several weeks for the kidney to recover from interrupting the blood supply [18].
In our case, prompt identification and quick repair of the injured right renal artery significantly reduced the risk of renal damage. The evidence is that 1) the right kidney recovered quickly and produced ample urine immediately after the vascular repairconsequently the warm ischemia time was short. 2) The right kidney continued to produce urine even when the patient was on dialysis. 3) Final CT scan before death showed the right renal artery patent and with a well perfused right kidney. 4) Postmortem exam showed the right kidney to be grossly normal.
Table 1:Selected studies reported on the outcomes of SMA injuries.
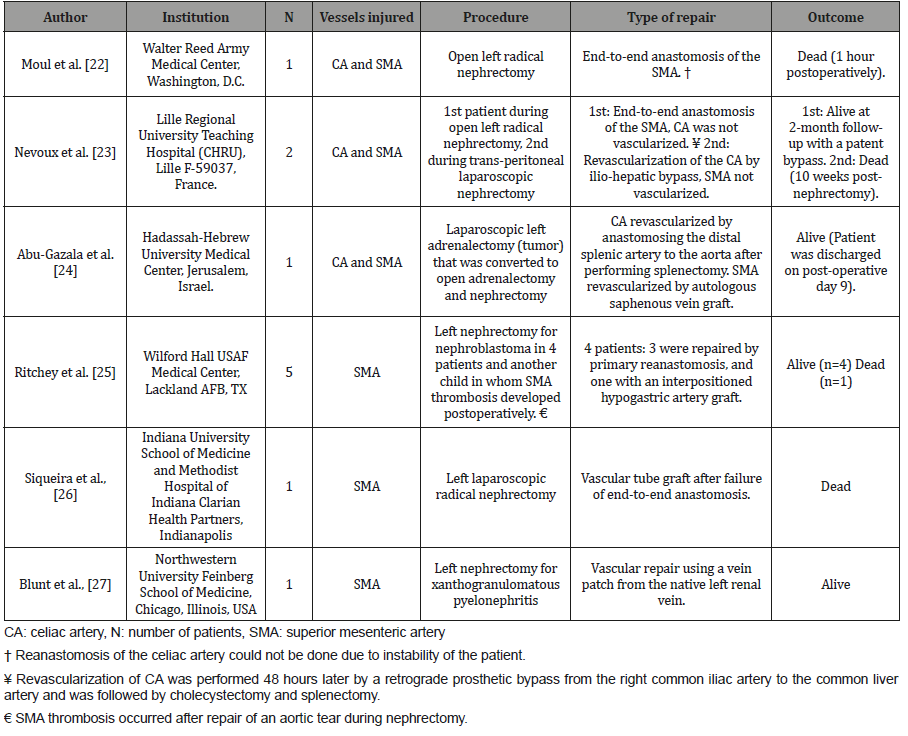
The SMA originates on the anterior surface of the aorta at the level of the L1 vertebrae, approximately 1 cm inferior to the celiac artery and superior to the renal arteries. The left renal vein runs directly between the aorta and the takeoff of the SMA [19]. Brunet C, et al. [20] reported the distance between the ostium of the left renal artery and SMA, and between the ostium of the celiac artery and the SMA to be 11.3 mm (3-22 mm) and 3.8 mm (1-11 mm), respectively [20]. Consequently, it is not uncommon that SMA injuries be associated with concomitant celiac artery injuries. However, injuries of the celiac artery are rarely life threatening than SMA ones since vital organs supplied by the celiac artery as the liver have dual blood supply and the robust collateral circulation of the proximal gut [21]. Injuries to the SMA may lead to a fatal outcome despite apparently adequate vascular repair. We have summarized pertinent studies on the outcomes of SMA injuries in (Table 1).
Conclusion
We described the case of a vascular injury to the right renal artery and the SMA during a robotic left radical nephrectomy. We immediately identified both injuries and intraoperative repair for both injuries was performed by vascular surgery team. The kidney survived the repair however the small bowel did not and the patient died secondary to bowel gangrene. Immediate identification and prompt repair to the injured renal artery reduced the ischemia time and reduced the risk of acute tubular necrosis. Repair of the SMA using a PTFE graft is not a guarantee that the bowel will survive.
Acknowledgement
None.
Conflict of Interest
No conflicts of interest.
References
- https://www.jointcommission.org/assets/1/18/UP_Poster1.PDF.
- https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/4 80UrologyCore2019-TCC.pdf?ver=2019-06-29-145847-003.2
- Thiel DD, Patel VR, Larson T, Lannen A, Leveillee RJ (2013) Assessment of robotic simulation by trainees in residency programs of the Southeastern Section of the American Urologic Association. J Surg Educ 70(5): 571-577.
- Rocha R, Fiorelli RK, Buogo G, Rubistein M, Mattos RM, et al. (2016) Robotic-assisted laparoscopic prostatectomy (RALP): a new way to training. J Robot Surg 10(1): 19-25.
- Lucas SM, Gilley DA, Joshi SS, Gardner TA, Sundaram CP (2011) Robotics training program: evaluation of the satisfaction and the factors that influence success of skills training in a resident robotics curriculum. J Endourol 25(10): 1669-1674.
- Schroeck FR, de Sousa CA, Kalman RA, Kalia MS, Pierre SA, et al. (2008) Trainees do not negatively impact the institutional learning curve for robotic prostatectomy as characterized by operative time, estimated blood loss, and positive surgical margin rate. Urology 71(4): 597-601.
- Schommer E, Tonkovich K, Li Z, Thiel DD (2016) Impact of Resident Involvement on Robot-Assisted Radical Prostatectomy Outcomes. J Endourol 30(10): 1126-1131.
- Lescay HA, Tuma F (2019) Anatomy, Abdomen and Pelvis, Ureter. StatPearls, USA
- Lopez PP, Gogna S, Khorasani Zadeh A (2019) Anatomy, Abdomen and Pelvis, Duodenum. StatPearls, USA
- Kara E, Ozturk NC, Ozgur A, Yildiz A, Ozturk H (2011) Ectopic kidney with varied vasculature: demonstrated by CT angiography. Surg Radiol Anat 33(1): 81-84.
- Kavoussi LR SM, Gill I (2012) Laparoscopic Surgery of the Kidney. In: Wein AJ, Kavoussi LR, Novic AC Phildelaphia (eds), Campbell-Walsh Urology. 10th (edn), 2(55): 1628-1669.
- Ozkan U, Oguzkurt L, Tercan F, Kizilkilic O, Koc Z, et al. (2006) Renal artery origins and variations: angiographic evaluation of 855 consecutive patients. Diagn Interv Radiol 12(4): 183-186.
- Sapte E, Iliescu D (2004) Double renal arteries originating from the aorta. Surg Radiol Anat 26(6): 474-479.
- Lee H, Song BD, Byun SS, Lee SE, Hong SK (2019) Impact of warm ischaemia time on postoperative renal function after partial nephrectomy for clinical T1 renal cell carcinoma: a propensity score-matched study. BJU Int 121(1): 46-52.
- Mir MC, Pavan N, Parekh DJ (2016) Current Paradigm for Ischemia in Kidney Surgery. J Urol 195(6): 1655-1663.
- Zabell JR, Wu J, Suk Ouichai C, Campbell SC (2017) Renal Ischemia and Functional Outcomes Following Partial Nephrectomy. Urol Clin North Am 44(2): 243-255.
- Laven BA, Orvieto MA, Chuang MS, Ritch CR, Murray P, et al. (2004) Renal tolerance to prolonged warm ischemia time in a laparoscopic versus open surgery porcine model. J Urol 172(6 Pt 1): 2471-2474.
- Ramsay AG, D Agati V, Dietz PA, Svahn DS, Pirani CL (1983) Renal functional recovery 47 days after renal artery occlusion. Am J Nephrol 3(6): 325-328.
- Shaikh H, Khorasani Zadeh A (2019) Anatomy, Abdomen and Pelvis, Superior Mesenteric Artery. StatPearls. Treasure Island (FL).
- Brunet C, Moutardier V, Di Marino V (1993) [The so-called “celiomesenteric button-hole” region, an anatomico-surgical study]. J Chir (Paris)130(2): 70-73.
- Long CA, Kwolek CJ, Watkins MT (2013) Vascular Trauma. In: Creager MA, Beckman JA, Loscalzo J (eds), Vascular Medicine: A Companion to Braunwald’s Heart Disease 2nd (edn). pp. 739-754.
- Moul JW, Foley JP, Wind GG, Rubin S, Coffey JA (1991) Celiac axis and superior mesenteric artery injury associated with left radical nephrectomy for locally advanced renal cell carcinoma. J Urol 146(4): 1104-1107.
- Nevoux P, Zini L, Villers A, Boleslawski E, Nunes B, Zerbib P (2008) Celiac axis and superior mesenteric artery: danger zone for left nephrectomy. J Endourol 22(11): 2571-2574.
- Abu Gazala S, Schlager A, Elazary R, Keidar A, Appelbaum L, et al. (2010) Revascularization of the celiac and superior mesenteric arteries after operative injury using both splenic artery and saphenous graft. Ann Vasc Surg 24(5): 693 e1-4.
- Ritchey ML, Lally KP, Haase GM, Shochat SJ, Kelalis PP (1992) Superior mesenteric artery injury during nephrectomy for Wilms’ tumor. J Pediatr Surg 27(5): 612-615.
- Siqueira TM, Kuo RL, Gardner TA, Paterson RF, Stevens LH, et al. (2002) Major complications in 213 laparoscopic nephrectomy cases: the Indianapolis experience. J Urol168(4 Pt 1): 1361-1365.
- Blunt LW, Matsumura J, Carter MF, Gonzalez CM, Smith ND (2004) Repair of superior mesenteric artery ligation during left nephrectomy with a native renal vein patch. Urology 64(2): 377-378.
-
Mahmoud I Khalil, Benjamin Schurhamer, Mohamed H Kamel. Vascular Injuries During A Robotic Left Radical Nephrectomy: Case Report. Annal Urol & Nephrol. 1(4): 2019. AUN.MS.ID.000518.
-
Radical nephrectomy, Robotic, Nephrectomy, Uperior mesenteric artery, Renal artery, Contralateral, Injury hemodynamics, Urologic, Oncology clinic, Injury, Surgery
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






