 Short Communication
Short Communication
Evaluation of the 29 MHz Micro-Ultrasound Imaging for Prostate Cancer Diagnosis and Treatment
Whitney Stanton1*, E. David Crawford2, Paul Arangua1, Gretchen Hoyer1 and Priya N Werahera1
1University of Colorado Anschutz Medical Campus, Aurora, CO, USA
2University of California San Diego, San Diego, CA, USA
Whitney Stanton, Department of Pathology, University of Colorado Anschutz Medical Campus, Mail Stop 8104, P.O. Box 6511, Aurora, CO 80045, USA.
Received Date: June 16, 2019; Published Date: June 27, 2019
Abstract
We describe our experience with four patients undergoing cryotherapy for treatment of prostate cancer. Micro-ultrasound was utilized in conjunction with standard transrectal ultrasound to intraoperatively assess lesions. In addition, the results were compared to preoperative mpMRI in several patients. The use of micro-ultrasound has been evaluated in clinical trials to include real-time imaging in the clinic for biopsies and fusion with prior mpMRI. We evaluated the technique in men with known prostate cancer undergoing cryoablation. Micro-ultrasound has the potential to replace the current clinical methods for targeted prostate biopsies and improve intraoperative monitoring.
Keywords: Exact Imaging; mpMRI; Conventional ultrasound; Micro-ultrasound; Biopsy; Prostate cancer
Abbreviations: mpMRI: Multiparametric Magnetic Resonance Imaging; PSA: Prostate Specificantigen; PIRADS v2: Prostate Imaging and Reporting Data System Version 2; TRUS: Trans-Rectal Ultrasound
Introduction
Prostate cancer is the most common form of malignancy and the second leading cause of cancer death in men in the United States. In 2019, approximately 174,650 cases of prostate cancer will be diagnosed [1]. Although a serious disease, most men will not die from prostate cancer. The use of the prostate specific antigen (PSA) blood test and trans-rectal ultrasound guided (TRUS) biopsy have resulted in over-diagnosis and overtreatment of clinically insignificant prostate cancer while also missing high-risk clinically significant tumors [2]. Clinically significant prostate cancer is defined as a lesion with high-grade prostate cancer (Gleason Score ≥ 7) or volume ≥ 0.5 cc. Therefore, it is important to identify men who have clinically significant prostate cancer who would benefit from treatment as well as identify men with low-risk tumors who would benefit from a more conservative approach such as active surveillance.
The use of multiparametric magnetic resonance imaging (mpMRI) in conjunction with the Prostate Imaging and Reporting Data System version 2 (PIRADS v2) allows physicians to target lesions in the prostate for biopsy [2]. PIRADS v2 assigns one composite score that indicates risk of clinically significant prostate cancer on mpMRI. A PIRADS score of 3, 4, or 5 designates intermediate, high, or very high risk of clinically significant prostate cancer, respectively. Men with a PIRADS score ≥ 3 are recommended for prostate biopsy, but a PIRADS score ≤ 2 cannot rule out clinically significant prostate cancer. Conventionally, a patient undergoes mpMRI and the urologist uses the mpMRI report to guide prostate biopsies in-office using a real-time urologic ultrasound, which operates at 6-9 MHz. The ExactVu™ Micro-Ultrasound system (ExactVu™ Micro-Ultrasound, Exact Imaging, Markham, Canada) is a new 29 MHz prostate imaging technique which provides a realtime imaging of cancer lesions at a high resolution of 70 microns [3]. In a study comparing high resolution micro-ultrasound imaging to mpMRI, micro-ultrasound imaging provided similar sensitivity to clinically significant prostate cancer as mpMRI. Micro-ultrasound imaging provides a real-time, high-resolution ultrasound platform and can be used to guide prostate biopsies in-office with improved imaging resolution compared to conventional urologic ultrasound, making it more time and cost effective. Herein, we describe our findings of micro-ultrasound imaging compared to preoperative mpMRI for the diagnosis of cancer lesions. Micro-ultrasound was employed as an adjunct in addition to standard-of-care TRUS in four men undergoing primary and salvage cryotherapy.
Discussion
This study received institutional review board approval under COMIRB #19-139. Four patients underwent cryotherapy for treatment of non-metastatic prostate cancer. Each patient had confirmatory TRUS biopsy pathology, three of the four men had prior mpMRI, and all men had micro-ultrasound before, during, and after cryotherapy in addition to the standard-of-care transrectal ultrasound. All cases were performed according to the same surgical protocol and by the same surgeon. Two freeze thaw cycles were completed and were monitored via ultrasound. The entire prostate gland was treated. The cryotherapy probes were placed through the cryotherapy template under ultrasound guidance [4].
Case 1
This patient was a 73-year-old male with no family history of prostate cancer. He had previous cyberknife radiotherapy in 2011 for Gleason grade group 3 (4 + 3 = 7) prostate cancer involving seven out of 26 biopsy cores. Cyberknife radiotherapy is a form of imageguided stereotactic body radiation therapy [5]. After cyberknife radiotherapy, his PSA nadired to 0.4 ng/ml, but slowly increased to 2.6 ng/ml prompting a PET-CT scan in August 2018. The PETCT revealed a centrally located focus of increased tracer uptake within the inferior prostate, likely compatible with malignancy. There was no evidence of bony metastases. In September 2018, the patient underwent a 20-core TRUS biopsy and was found to have Gleason grade group 3 (4 + 3 =7) in 1 of 20 cores and Gleason grade group 2 (3 + 4 = 7) in 1 of 20 cores involving 15 and 30% of each core, respectively. The mpMRI demonstrated a 1.6 cm diffusion restricting early enhancing lesion in the central gland near the apex and was concerning for disease recurrence. The prostate volume was 32 cc. During his cryotherapy procedure, three probes were used: two on the left and one on the right. The prostate volume was 49.7 g. The micro-ultrasound identified suspicious lesions bilaterally at the apex, consistent with the mpMRI.
Case 2
This patient was a 67-year-old male with history of a urethral stricture. An elevated PSA of 6.77 ng/ml prompted a TRUS biopsy that revealed Gleason grade group 3 (4 + 3 = 7) prostate cancer with multifocal perineural invasion. He had a negative CT of the abdomen and there was no evidence of metastatic disease in the abdomen or pelvis. A mpMRI of the prostate revealed a PIRADS 4 nodule (9 mm hypointense) in the lateral left peripheral zone in the mid aspect of the gland with acute restriction and a PIRADS 3 nodule (2.3 cm) in the anterior left transition zone from mid to base that predominantly had well-circumscribed boundaries, but demonstrated asymmetric enhancement with acute restriction. During his cryotherapy procedure, seven probes were used: four on the left and three on the right. The prostate volume was 50.3 g. The micro-ultrasound clearly visualized seminal vesicle invasion and extra-prostatic extension on left side, consistent with his biopsy findings.
Case 3
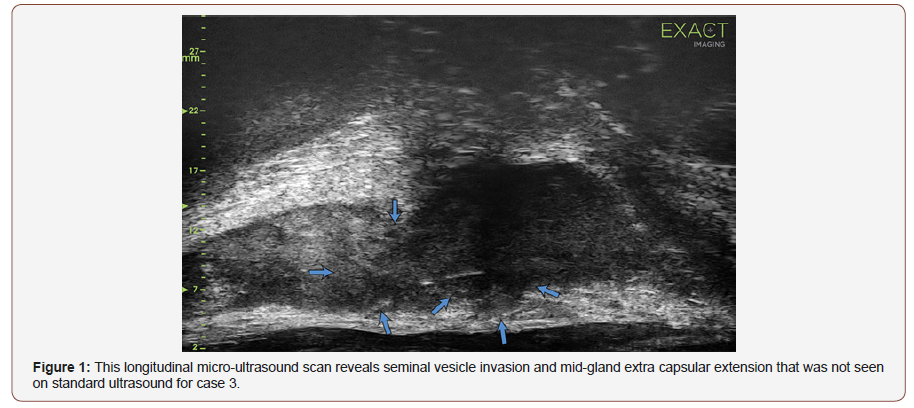
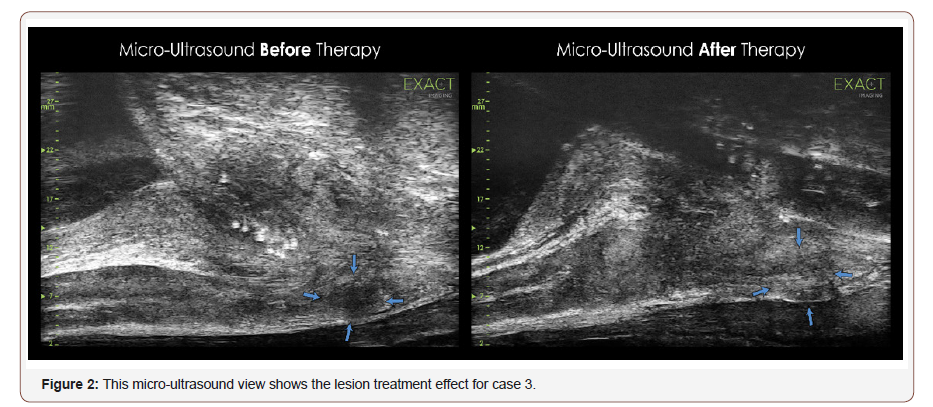
A 59-year-old male with a history of brachytherapy and biopsy proven recurrence underwent salvage cryotherapy. A TRUS biopsy revealed Gleason grade group 2 (3 + 4 = 7) prostate cancer with perineural invasion. A CT of the abdomen and pelvis showed no evidence of soft tissue extension, suspicious lymph nodes or metastatic lesion involving the skeletal anatomy. During his cryotherapy procedure the prostate volume measured was only 11 g. The micro-ultrasound showed clear extra-capsular and seminal vesicle invasion before cryotherapy shown in the figure with arrows. Brachytherapy seeds were visible with posttreatment changes. Left lateral to midline lesions were observed with suspected extraprostatic extension, especially at the midline (Figure 1&2).
Case 4
This patient was a 76-year-old male with Gleason grade group 5 (4 + 5 = 9) on TRUS biopsy. His mpMRI revealed a prostate volume of 27.1 g and post-treatment changes in the prostate with a suspicious area of signal in the left posterior peripheral zone at the base of the gland (PI-RADS 4). The CT of the abdomen and pelvis was negative for metastases. A whole-body Sodium Fluoride PET/ CT Scan revealed uptake in c-spine and T/L region and possible rib lesions which were most likely old fractures. During the cryotherapy procedure, a total of four probes were placed: two on the left and two on the right. The prostate volume was 15.1 g. The micro-ultrasound showed brachytherapy seeds as well as the left base-to-mid cancer focus with clear extraprostatic extension. The apex bilaterally was suspicious for recurrence. The left base and left-mid showed obvious lesions with suspicion for extraprostatic extension, consistent with mpMRI.
Conclusion
We presented four cases where micro-ultrasound was used intraoperatively to assess the lesions and monitor cryotherapy. High resolution micro-ultrasound imaging and its ability to perform real-time targeted biopsies enables urologist-controlled in-office imaging and therefore does not require a hospital visit or radiologic involvement. Conventional trans-rectal ultrasound operates between 6-9 MHz, and has poor sensitivity while mpMRI is not real-time, misses significant multifocal disease, and has poor prediction of lesion size and shape. mpMRI drawbacks include significant financial and time costs to the patient and reliance on radiologist expertise in performing and interpreting the mpMRI and assigning a PIRADS score [3]. MRI-guided biopsies have a high physician learning curve, involve significant costs, and may lack reproducible results. Clinicians may use micro-ultrasound to improve screening and therapy protocols. Using micro-ultrasound as a screening modality may allow for patients to strictly adhere to active surveillance. Lesions identified by micro-ultrasound were consistent with corresponding mpMRI lesions. It provided additional intraoperative assessment of the prostate gland and capsule. As the micro-ultrasound images demonstrate, the spatial resolution is significantly improved over standard ultrasound without the significant changes to standard ultrasound procedure or cost seen when using mpMRI. In conclusion, micro-ultrasound has the potential to replace our current clinical methods for targeted biopsies and improve intraoperative monitoring.
Acknowledgement
We would like to thank Karen Costanten, Nadia Halstead, MD, Timothy Vanadurongvan, MD, and Luke Bidikov, MD.
Conflict of Interest
No conflicts of interest.
References
- Key Statistics for Prostate Cancer. American cancer society.
- Jonathan Sackett, Peter Choyke L, Baris Turkbey (2019) Prostate Imaging Reporting and Data System Version 2 for MRI of Prostate Cancer: Can We Do Better? American Journal of Roentgenology 212: 1244-1252.
- Eure G, Fanney D, Lin J, Wodlinger B, Ghai S (2019) Comparison of conventional transrectal ultrasound, magnetic resonance imaging, and micro-ultrasound for visualizing prostate cancer in an active surveillance population: A feasibility study. Can Urol Assoc J 13(3): E70-E77.
- Rosenkrantz AB, Ginocchio LA, Cornfeld D, Adam Froemming T, Rajan Gupta T, et al. (2016) Version 2 Lexicon: A Multicenter Study of Six Experienced Prostate Radiologists. Radiology 280(3):793-804.
- https://www.healthline.com/health/prostate-cancer/cyberknife-forprostate- cancer.
-
Whitney Stanton, E. David Crawford, Paul Arangua, Gretchen Hoyer, et al. Evaluation of the 29 MHz Micro-Ultrasound Imaging for Prostate Cancer Diagnosis and Treatment. Annal Urol & Nephrol. 1(3): 2019. AUN.MS.ID.000515.
-
Exact Imaging, mpMRI, Conventional ultrasound, Micro-ultrasound, Prostate cancer screening
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






