 Opinion
Opinion
Role of the Pharmacist in Managing Antidepressant Drug Interactions in the Solid Organ Transplant Population
Suzanne C Harris1* and Chaz Hyatt2
1Division of Practice Advancement and Clinical Education, University of North Carolina Eshelman School of Pharmacy, USA
2Department of Pharmacy, University of North Carolina Medical Center, USA
Suzanne C Harris, Division of Practice Advancement and Clinical Education, University of North Carolina Eshelman School of Pharmacy, USA.
Received Date:May 14, 2019; Published Date: May 23, 2019
Abstract
Major depressive disorder (MDD) is prevalent in solid organ transplant (SOT) patients and can lead to medication nonadherence and risk of rejection of the transplant organ. In addition, pharmacokinetic and pharmacodynamic drug interactions can pose challenges when using antidepressant and immunosuppressant medication concomitantly. Clinical pharmacists serve an important role in improving patient outcomes in SOT patients with depression by predicting and avoiding potential drug interactions, identifying when drug interactions warrant modifications, and educating patients and providers on safe and effective use of antidepressants in the transplant population.
Keywords:Pharmacist; Transplant; Depression; Antidepressant; Immunosuppressant; Interaction
Abbreviations: MDD: Major Depressive Disorder: SOT: Solid Organ Transplant; CYP: Cytochrome P450; SSRI: Selective Serotonin Reuptake Inhibitors; SNRI: Selective Serotonin and Norepinephrine Reuptake Inhibitors; DDI: Drug-Drug Interactions; CNS: Central Nervous System; PGP: P-glycoprotein; TCA: Tricyclic Antidepressants; MAOI: Monoamine Oxidase Inhibitors
Introduction
In transplant patients, Major Depressive Disorder (MDD) is the most prevalent psychiatric disorder and is associated with reduced quality of life, with prevalence rates of MDD in up to 25% of solid organ transplant (SOT) patients [1]. MDD can lead to medication nonadherence, which can cause rejection of the transplant organ. Additionally, antidepressant medications have a range of unique side effects and potential for pharmacokinetic and pharmacodynamic interactions. Many of the transplant medications are metabolized by cytochrome P450 3A4 enzymes, putting them at risk for pharmacokinetic drug interactions. In addition, these medications can have pharmacodynamic interactions with antidepressants. Corticosteroids can cause weight gain, leading to diabetes and obesity. Cyclosporine causes hypertension in 50% of kidney transplant patients. Tacrolimus and cyclosporine have been reported to have central nervous toxicity in one-third of patients. Corticosteroids, sirolimus, and cyclosporine have been associated with hyperlipidemia [2]. Hence, these interactions with antidepressants can result in altered concentrations of immunosuppressants or additive untoward side effects (Table 1).
Table 1 lists potential drug-drug interactions, notable side effects and precautions, and important points for consideration when combining antidepressants and immunosuppressant agents [2-5]. Clinical pharmacists have expertise in evaluating the clinical relevance of potential drug interactions and providing recommendations to reduce risks when warranted, ultimately helping to improve tolerability and clinical outcomes in SOT patients.
Table 1: Drug Interactions and Clinical Pearls of Antidepressants in SOT patients.
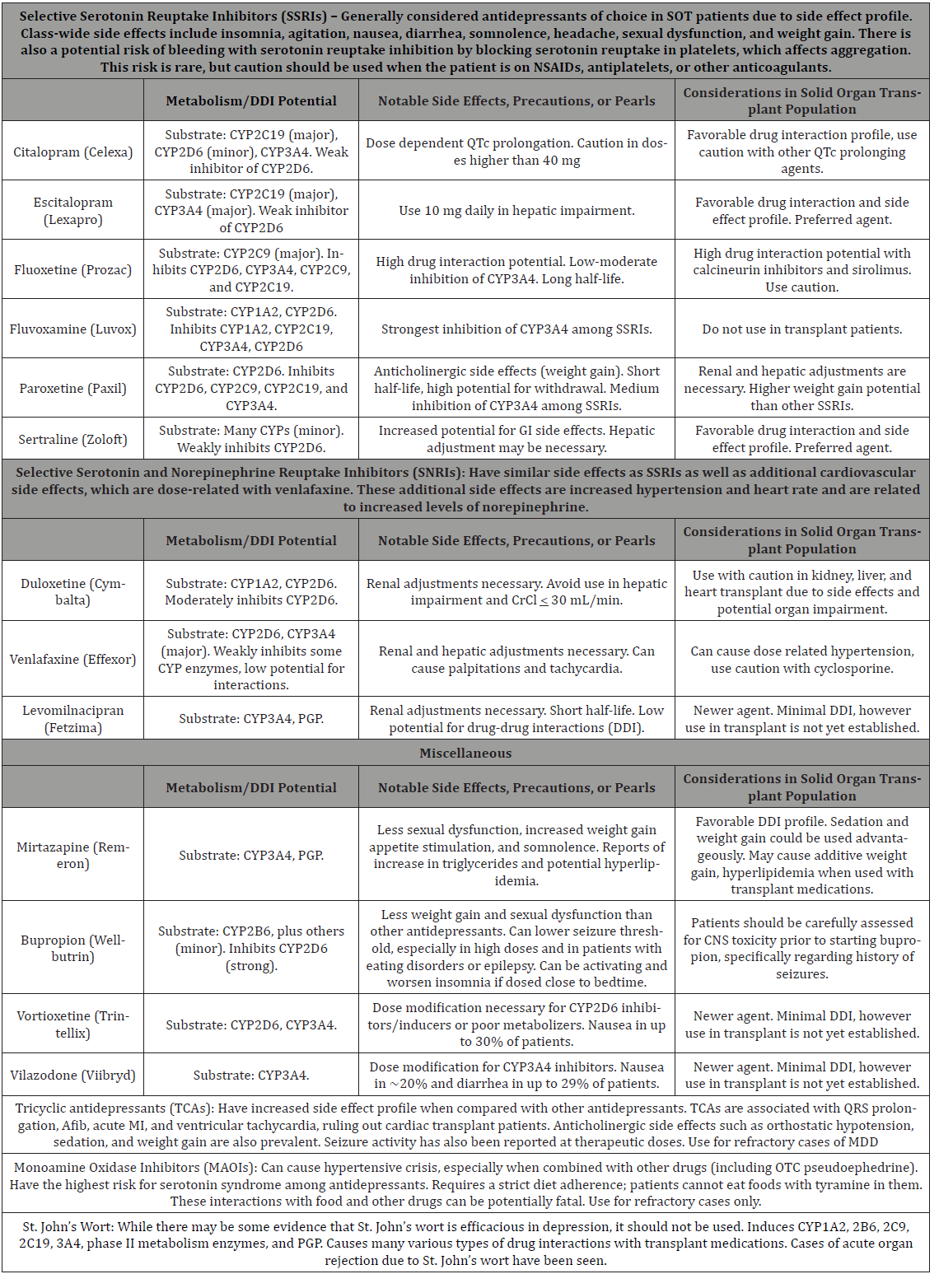
Discussion
Clinical pharmacists on consult liaison psychiatry, medical, or surgical teams actively participate in the medication management of solid organ transplant recipients, including the management of agents to treat depression in these patients. While most antidepressants are safe and well-tolerated, careful considerations of potential and clinically relevant interactions must be made in the recommendation of initial antidepressants or in modification of antidepressant regimens in SOT patients. Also, depending on where the patient is in the transplant process, different drug interactions may be more important. Pre-transplant concerns when initiating antidepressants may vary from clearance and metabolism of the antidepressant, or negative effects on the impaired organ. Posttransplant concerns when initiating antidepressants include inhibition or induction of immunosuppressants, or additive side effects to immunosuppressants regardless of metabolism; hence, careful monitoring of immunosuppressant levels or worsening of adverse effects may be necessary during the post-transplant phase. Newer antidepressants may have favorable CYP P450 drug interaction profiles when combined with immunosuppressants, however lack of established data in SOT patients limit their use.
Conclusion
Clinical pharmacists serve an important role in improving patient outcomes in SOT patients with depression by predicting and avoiding potential drug interactions, identifying when drug interactions warrant modifications, and educating patients and providers on safe and effective use of antidepressants in the transplant population.
Acknowledgement
None.
Conflict of Interest
No authors have any conflicts of interest to disclose.
References
- Dew MA, Kormos RL, Dimartini AF, Switzer GE, Schulberg HC, et al. (2001) Prevalence and risk of depression and anxiety-related disorders during the first three years after heart transplantation. Psychosomatics 42(4):300-313.
- Kim J, Phongsamran P, Park S (2019) Use of antidepressant drugs in transplant recipients. Prog Transplant 14(2):98-104.
- Kahl KG, Eckermann G, Frieling H, Hillemacher T (2019) Psychopharmacology in transplantation medicine. Prog Neuropsychopharmacol Biol Psychiatry 88:74-85.
- Teter CJ, Kando JC, Wells BG Major Depressive Disorder. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L (Eds.) Pharmacotherapy: A Pathophysiologic Approach, 10e, McGraw-Hill; New York, USA.
- Lexicomp Online. Lexi-Comp, Inc (Copyright 1978-2016).
-
Suzanne C Harris, Chaz Hyatt. Role of the Pharmacist in Managing Antidepressant Drug Interactions in the Solid Organ Transplant Population. Arch Phar & Pharmacol Res. 1(4): 2019. APPR.MS.ID.000518.
-
Pharmacist; Transplant; Depression; Antidepressant; Immunosuppressant; Interaction
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






