 Research Article
Research Article
Industrial Development of Hong Kong and its Environmental Impacts as Recorded by Sediment Pb Variation in Shenzhen Bay, South China
Caiying Zhong, Hong Su, Hongyu Yan and Shijun Jiang*
Institute of Groundwater and Earth Sciences, Jinan University, Guangzhou 510632, China
Shijun Jiang, Jinan University, Institute of Groundwater and Earth Sciences, Guangzhou, China.
Received Date: April 02, 2019; Published Date: April 17, 2019
Abstract
Lead (Pb) is a carcinogenic metal in environment mainly related to industry. To investigate the environmental impacts of Hong Kong economic development, we measured the Pb concentration in a sediment core from Shenzhen Bay with direct freshwater discharge from the city. The sediment chronological framework was established through comparing the variation pattern of Pb concentration between sediment core and instrumental data from a nearby monitoring station initiated in 1980s and was extrapolated to pre-1980 sediment record. The results show that the Pb concentrations in the sediment core fall between 40.0 and 109.8mg/kg. More importantly, the sediment Pb content shows a rapid increase from Year 1968 to 1987, then a continuous reduction until 2005 and leveled concentrations afterwards. This temporal variation coincides with Hong Kong’s industrial progress during 1950s through 1980s and strict enforcement of environmental protection policy issued in 1985, while is seemingly unrelated to Shenzhen’s urbanization soared after 1985, suggesting that the sediment Pb in Shenzhen Bay is mainly sourced from the industrial development in Hong Kong.
Keywords: Lead; Sediment; X-ray fluorescence (XRF); Grain size; Hong kong; Shenzhen bay
Introduction
Heavy metal pollution is caused by excessive accumulation of toxic heavy metal elements or metalloids in environment, which is not only harmful to natural ecosystems, but also indirectly threatens human health through the food chain [1]. It remains a challenging and hot topic for environmental scientists and oceanographers due to the wide sources, non-biodegradation, biological toxicity and bio-magnification of heavy metals [2-4]. The sources of heavy metals in marine environment are mainly divided into natural sources and human sources. The natural sources refer to those heavy metal elements transported into oceans via natural processes such as weathering, erosion and volcanic eruption. These natural processes speed up the movement of heavy metal from the earth’s crust into the aquatic environment through surface runoff and/or atmospheric deposition. This fraction almost stays stable in the environment, and thus often forms the environmental background values in scientific research. Instead, human activities, particularly industrial development, have been the main source of heavy metals. Most heavy metals associated closely with urbanization and industrial development are inevitably discharged into marine environment even the strictest policies are implemented. The main sources come from mining and smelting industries and industrial wastewater [5,6]. The metal elements originally exist in the Earth’s crust. Through chemical and physical weathering, heavy metals in particles or complexes are transported via runoff, fluvial and atmosphere processes, and enter lakes and/or oceans and finally accumulate in sediments [6,7]. So, sediments are the final destination of heavy metals, and changes in hydrological conditions affect their migration and deposition processes.
In most studies on heavy metal contamination, lead (Pb) has been included as an important toxic element for its high toxicity and high concentration. During the past several decades, environmental pollution caused by Pb accumulation has strongly threatened the health of ecosystems as well as human beings [1,8,9]. Because of the extent and seriousness of Pb contamination, the Chinese government has included Pb in the list of environmental priority pollutants [10]. Pb is an indispensable industrial raw material for building industry, manufacturing and military research, such as the production of storage batteries, X-ray protection equipment and wires and cables due to its soft and ductile nature [11]. Its wide use in industry inevitably accelerates the accumulation rate in the terrestrial and marine environments. Therefore, it is of great significance to investigate the temporal variation of sediment Pb concentration for assessing pollution level, inferring its sources, and guiding future environmental protection plans.
Shenzhen Bay, also known as Deep bay in Hong Kong, refers to the waters between Shenzhen Special Economic Zone and Hong Kong Special Administrative Region of China (Figure 1). Domestic wastewater and industrial wastewater from Shenzhen City flow directly into this bay through Shenzhen River and other small rivers. There is a mangrove forest growing in the intertidal zone of Shenzhen Bay (Futian Mangrove Reserve). Mangrove wetlands are considered as a kind of valuable ecosystem that can remove nutrients and other organic and inorganic pollutants [12- 14]. Previous studies have shown that the mangrove forests in Shenzhen Bay have been polluted by different heavy metals mainly from electronic and chemical industries and domestic sewage through surface runoff, wastewater discharge, vehicle emissions and maritime transportation [15-18].
As Hong Kong entered the industrialization period in the 1950s during which its environmental protection supervision was not strict enough [19-21], its environment underwent increasingly severe pollution by heavy metals, and its Pb pollution was mainly from electroplating and fuel combustion [15,20]. Shenzhen has experienced rapid urban and industrial development in coastal areas since 1980s [22]. How has the economic development of these two cities contributed to the heavy metal pollution of Shenzhen Bay provides important inference for their development pattern.
Here we investigate the temporal variation in Pb concentration during the past half century obtained in a sediment core from Shenzhen Bay, and compare with the monitoring data from a nearby observatory. We then compare the Pb variation pattern with the industrialization process of Hong Kong and Shenzhen cities to infer their relative contribution of Pb to the Shenzhen Bay sediments.
Materials and Methods
Study area and sample collectionb
Shenzhen Bay is located in the eastern part of the Pearl River Estuary, separating Shenzhen in the north from Hong Kong in the south (Figure 1). It is a semi-enclosed small bay directly connected with the coastal waters. The bay covers an area of about 100km2, 17km long and 4-10km wide. It is also connected with several rivers, such as Shenzhen River, Feng tang River and the Pearl River Estuary. The Shenzhen River flows into Shenzhen Bay from east to west and forms the boundary between Hong Kong and Shenzhen. The Shenzhen Bay has a flat seabed, and pollutants discharged from Shenzhen, Hong Kong, Shenzhen River and Pearl River can easily spread across the entire bay area [23]. Shenzhen Bay lies in the subtropical marine monsoon climate zone in the East Asian monsoon region. It has an annual average temperature of 22.4 °C, relative humidity of ~80%, rainfall of 1700-1900mm with a rainy season from April to October, and evaporation of 1500-1800mm. There are annually 2-4 typhoon landings during summer and autumn. The tide in Shenzhen Bay is semidiurnal with an average tidal level of 0.33m (the highest is 2.66m and the lowest is 1.56m), and the average wave height is 0.9m [24].
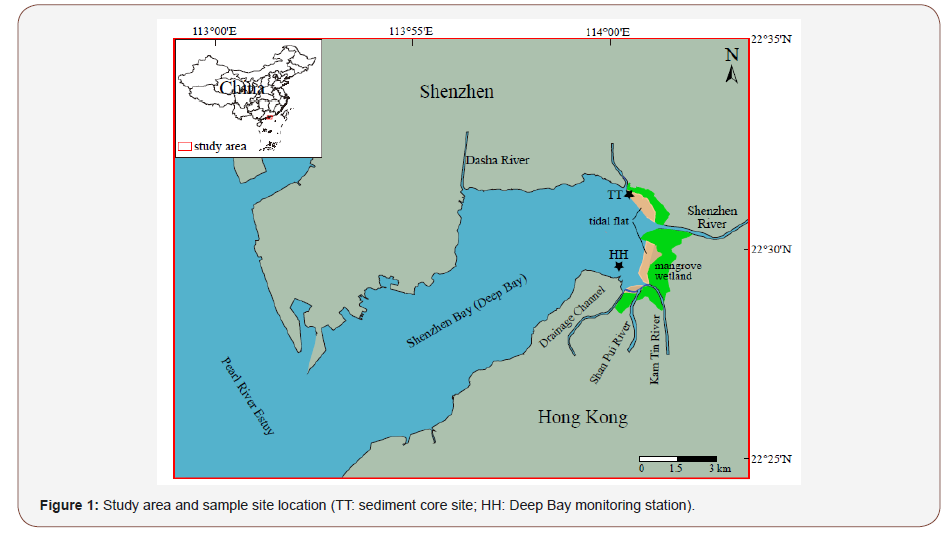
A 50-cm-long sediment core was obtained on December 26, 2014 with a 7.5-cm-diameter PVC pipe pressed into the tidal flat sediments of Shenzhen Bay (Figure 1; 22°31’18.85 “N, 114°0’57.02” E). After retrieved, the sediment core was covered with clear water and a clear mud-water interface was observed, which indicates that the core was not disturbed during coring operation. The core was transported to the laboratory in a vertical state and kept at 4 °C in a refrigerator before processing. The core was split and sliced at 1-cm interval. Each sample was weighed before and after freezedrying to derive the water content, and then stored in a black selfsealed bag until further analysis.
Pb content analysis
The main heavy metal measurement methods include X-ray fluorescence spectrometer (XRF), atomic absorption spectrophotometer (AAS), and inductively coupled plasma mass spectrometry (ICP-MS). Previous studies have compared the reliability of these methods and concluded that the three commonly used technologies maintain a high level of data consistency [25- 28]. X-ray fluorescence spectrometer (XRF) has been widely used in soil and sediment analyses due to its particular advantages, such as simple sample preparation, non-destructiveness, high precision, and good repeatability and reliability [29,30]. As such, an XRF device (EDX-6000B, Skyray Instrument Inc.) with a 10-chamber automatic sampler was used to determine sediment Pb concentration.
Prior to analysis, the freeze-dried samples were thoroughly mixed and then ground with an agate mortar to obtain a powder which can pass through a 200-mesh sieve. Then ~0.8 g of powder was weighted and pressed into a tablet using a manual hydraulic tablet maker under a pressure of 20 MPa. Each tablet was repeatedly measured 10 times, and the average was calibrated with a Chinese national stream sediment standard (GBW07305a). During the measurement, the variation coefficient was maintained within 7%.
Grain size analysis
A laser particle size analyzer (MasterSizer 3000, Malvern) was used for particle size analysis. Prior to analysis, 0.2~0.4 g of freezedried samples were weighed and placed in a 50 ml centrifuge tube, adding 10 ml of 30% H2O2 to remove organic matter and 10 ml of 10% HCl to remove carbonate. After centrifugation, the supernatant was removed. The residue was washed more than 3 times until the supernatant was neutral. The resulting residue was added 10 ml of 0.5 mol/L sodium hexametaphosphate [(NaPO3)6] solution, soaked for 24 h, then centrifuged, removed the supernatant, freeze-dried the remaining precipitate and then put it into the instrument for particle size measurement. The detection range of the instrument is 0.01~3500 μm. Each sample was measured 5 times and the frequency distribution curve of 5 times must keep consistent. Finally, the granularity percentage chart was made with a C2 software.
The chronological sequence of sediment core
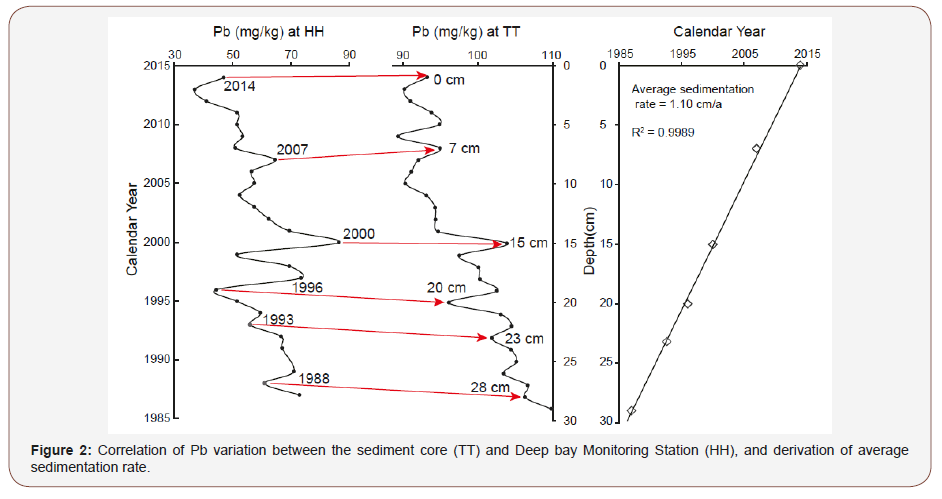
The chronological sequence was established for the sediment core through comparing the variation pattern of Pb content in the sediment with that provided by the Deep Bay Monitoring Station (HH; Figure: 1), which is located very close to our coring site in Shenzhen Bay and has continuously determined and recorded the sediment Pb content since 1987 (https://www.epd.gov.hk). Then the average sedimentation rate was used to derive the age of each sample of the entire sediment core.
Results and Discussion
Chronology
As shown in Figure 2, the variation pattern of sediment Pb content (TT) shows close similarity to that of the Deep Bay Monitoring Station (HH). This similarity is used for the first time to construct the chronological frame of the sediment core. By using the six correlation points (left panel, Figure 2), an average sedimentation rate of 1.10 cm/a is derived (right panel, Figure 2). Based on the average rate, the age of each sample is obtained according to its depth, and thus our 50-cm-long sediment core represents a record of ~45 years spanning from 1968 through 2014.
Temporal variation in sediment Pb concentration and its implications
The sediment Pb concentration ranges from 40.0 to 109.8 mg/ kg with an average value of 95.5 mg/kg. As shown in Figure 2, the Deep Bay monitoring station (HH) shows systematically lower Pb concentrations than at the tidal flat site (TT), which can be attributed to the different methods used to measure the Pb content. The current study used a full element measurement method (XRF) that can detect all element fractions including those trapped in insoluble siliceous minerals, while the Deep Bay Station used the U.S. Environmental Protection Agency (USEPA) method that only measures the dissolved metal fractions in sediments extracted and/ or leached by acids; the latter method generally recovers most of the heavy metal contents but does not recover the metals locked in the insoluble silicates [31]. Despite the difference in concentration value, both datasets show a similar trend with three prominent intervals recognized: rapid increase during 1968-1987, continuous decrease during 1987-2005, and relatively steady stage during 2005-2014 (Figure 3).
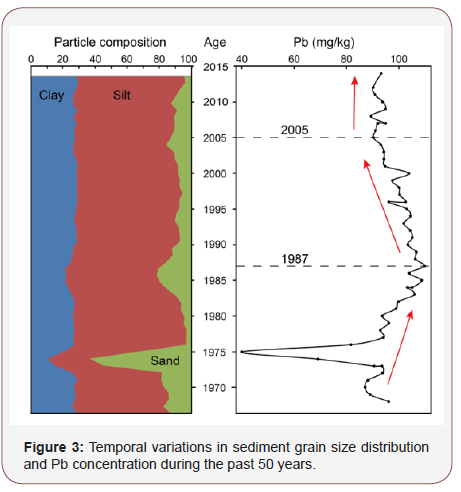
There is a rapid increase trend in the sediment Pb concentration during interval 1968-1987. The sharp decrease from 1975 to 1977 with Pb level dropped to half of its previous values has also been observed in other studies with reservoir sediments, which is attributed to dilution by terrestrial input from unusually higher precipitation [32]. This is because that particle size determines the mobility of particles and their associated pollutant concentrations, and heavy metals are favorably adsorbed in particulate matter with smaller particle sizes, so sands have a dilution effect on heavy metal concentration when heavy rainfalls and floods easily transport these large particles into the bay [33,34]. It is worth noting that sands sharply increased from 1975 to 1977 reaching 55% of the total content, and this is consistent with more heavy rain events and longer rain periods during this time interval (China Meteorological Data Network, http://data.cma.cn).
The Pb increase during 1968-1987 seems to be more influenced by Hong Kong’s economic development. Before the Shenzhen Special Economic Zone was established in 1980, Shenzhen was a little undeveloped village with a self-sufficient economy of small-scaled agriculture and seafood, and there was no substantial industrial development until the late 1980s when China began to carry out economic reform and opening up [19,35]. In contrast, Hong Kong entered its industrialization era in 1950s with its industry peaking in the 1980s. During this era, Hong Kong had developed a massive manufacturing industry (e.g., electronic product enterprises) and transportation system consuming a large amount of leaded gasoline [36], while unfortunately, no environmental protection policy was implemented strictly and effectively [22].
The massive discharge of industrial wastewater and fuel combustion led to the higher sediment Pb concentrations (average: 92.8 mg/kg) in Hong Kong’s tidal flat than that of the sediment Pb background value in Guangdong coastal zone [37]. Large-scale heavy metal pollution in Shenzhen Bay has appeared since the 1950s [20,21,38], and the difference in timing of extensive industrial development between Shenzhen and Hong Kong suggests that Pb was mainly sourced from Hong Kong.
Interval 1987-2005 marks a steady decline in sediment Pb concentration. The beginning of this period coincides with the soar away industrial growth of the Chinese Pearl River Delta Region including two megacities (Guangzhou & Shenzhen), and Hong Kong’s industrial adjustment and transformation to adapt to economic restructuring, the latter which includes tertiary industrial boom and subsequent de-industrialization processes [39]. In addition, as the surrounding environment deteriorated, raising public awareness of environmental protection had urged the establishment of the Hong Kong Environmental Protection Agency (HKEP) in 1986, which has played a decisive role in regulating discharge of waste water, waste gas and waste residues and managing environmental restoration. The reduction in sediment Pb level during this time period likely resulted from Hong Kong’s economic restructuring and governmental and public involvement in waste control, while the economic growth in Pearl River Delta Region did not counteract the mitigation efforts in Hong Kong. The alleviated heavy metal pollution was also observed in Hong Kong’s Victoria Harbor [20].
The leveled sediment concentration during interval 2005-2014 suggests that environmental protection and industrial development have reached an equilibrium state. As the surrounding areas of Shenzhen Bay house more and more manufacturing industry and population, the rapid urbanization at the current technology level progresses unavoidably at the price of environmental pollution from industrial and municipal wastewaters [40,41], even if effective measures are taken, such as prohibited use of leaded fuel and strict sewage treatment. This indicates that the concentration of heavy metals in sediments from coastal waters, such as Shenzhen Bay, will not return to pre-pollution levels, but governmental regulation and public involvement have demonstrated great strength in environmental restoration, improvement and preservation.
Conclusion
The reconstructed sediment Pb concentrations in Shenzhen Bay during the past 50 years fall between 40.0 and 109.8 mg/ kg with an average of 95.5 mg/kg. Its temporal variation shows a rapid increase during 1968-1987, continuous decrease during 1987-2005, and leveled concentration during 2005-2014. This pattern closely matches the Hong Kong’s industrial progress, which developed extensively from 1950s and peaked during 1970s and 1980s periods. The establishment of the Hong Kong Environmental Protection Agency in 1986 seems to have reversed the increasing Pb trend. The close match indicates that the source of Pb in the sediment core in Shenzhen Bay is mainly from the discharge of industrial wastes in Hong Kong.
Acknowledgments
We thank Anhong Zhang, Jun Liu, Jiangang Zhao, and Lin Xiao from Jinan University for help with the sediment coring, and Yasu Wang for discussion on an earlier draft of this manuscript. The study is financially supported by the National Science Foundation of China (41672004 & 41806134).
References
- Gambelunghe G, Sallsten Y, Borné N, Forsgard B, Hedblad P, et al. (2016) Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environ Res 149: 157-163.
- Tchounwou PB, Yedjou VG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101: 133-164.
- Lychagin M, Chalov S, Kasimov N, Shinkareva G, Jarsjö J, et al. (2017) Surface water pathways and fluxes of metals under changing environmental conditions and human interventions in the Selenga River system. Environmental Earth Sciences 76: 1.
- Chen M, Qin X, Zeng G, Li J (2016) Impacts of human activity modes and climate on heavy metal “spread” in groundwater are biased. Chemosphere 152: 439-445.
- Foster IDL, Charlesworth SM (1996) Heavy metals in the hydrological cycle: trends and explanation. Hydrological processes 10(2): 227-261.
- Callender E (2003) Heavy metals in the environment-historical trends. Treatise on geochemistry 9: 612.
- Andrea I Pasquini, Pedro J Depetris (2012) Hydrochemical considerations and heavy metal variability in the middle Paraná River. Environmental Earth Sciences 65(2): 525-534.
- Gu YG, Ning JJ, Ke CL, Huang HH (2018) Bioaccessibility and human health implications of heavy metals in different trophic level marine organisms: A case study of the South China Sea. Ecotoxicology and environmental safety 163: 551-557.
- Gati G, Pop C, Brudaşcă F, Gurzău AE, Spînu M (2016) The ecological risk of heavy metals in sediment from the Danube Delta. Ecotoxicology 25: 688-696.
- Gasperi J, Garnaud S, Rocher V, Moilleron R (2008) Priority pollutants in wastewater and combined sewer overflow. Sci Total Environ 407(1): 263-272.
- Settle DM, Patterson CC (1980) Lead in albacore: guide to lead pollution in Americans. Science 207(4436): 1167-1176.
- Robertson AI, Duke N (1987) Mangroves as nursery sites: comparisons of the abundance and species composition of fish and crustaceans in mangroves and other nearshore habitats in tropical Australia. Marine Biology 96(2): 193-205.
- DC Donato, JB Kauffman, Murdiyarso D, Kurnianto S, Stidham M, et al. (2011) Mangroves among the most carbon-rich forests in the tropics. Nature geoscience 4: 293-297.
- Li D, Xu Y, Li Y, Wang J, Yin X, et al. (2018) Sedimentary records of human activity and natural environmental evolution in sensitive ecosystems: A case study of a coral nature reserve in Dongshan Bay and a mangrove forest nature reserve in Zhangjiang River estuary, Southeast China. Organic Geochemistry 121: 22-35.
- Xu S, Lin C, Qiu P, Song Y, Yang W, et al. (2015) Tungsten-and cobaltdominated heavy metal contamination of mangrove sediments in Shenzhen, China. Mar Pollut Bull 100(1): 562-566.
- Huang X, Li X, Yue W, Huang L, Li Y (2003) Accumulation of heavy metals in the sediments of Shenzhen Bay, South China, Huan jing ke xue= Huanjing kexue, 24(4): 144-149.
- Li R, Li R, Chai M, Shen X, Xu H, et al. (2015) Heavy metal contamination and ecological risk in Futian mangrove forest sediment in Shenzhen Bay, South China. Marine pollution bulletin 101(1): 448-456.
- Chai M, Shen X, Li R, Qiu G (2015) The risk assessment of heavy metals in Futian mangrove forest sediment in Shenzhen Bay (South China) based on SEM-AVS analysis. Mar Pollut Bull 97(1-2): 431-439.
- Chiu SWK, Ho Kong Chong, Lui Tai-lok (2018) City states in the global economy: Industrial restructuring in Hong Kong and Singapore, Routledge, UK.
- Tang CW, Ip CC, Zhang G, Shin PK, Qian PY, et al. (2008) The spatial and temporal distribution of heavy metals in sediments of Victoria Harbour, Hong Kong. Marine Pollution Bulletin 57(6-12): 816-825.
- Owen R, Sandhu N (2000) Heavy metal accumulation and anthropogenic impacts on Tolo Harbour, Hong Kong. Marine Pollution Bulletin 40(2): 174-180.
- Chen K, Jiao JJ (2008) Metal concentrations and mobility in marine sediment and groundwater in coastal reclamation areas: a case study in Shenzhen, China. Environ Pollut 151(3): 576-584.
- Ren H, Wu X, Ning T, Huang G, Wang J (2011) Wetland changes and mangrove restoration planning in Shenzhen Bay, Southern China. Landscape and Ecological Engineering 7(2): 241-250.
- Wang, B, Liao, B, Wang, Y, Zan, Q (2002) Mangrove forest ecosystem and its sustainable development in Shenzhen Bay, Science, Beijing, China.
- Trejos T, Koons R, Becker S, Berman T, Buscaglia J, et al. (2013) Crossvalidation and evaluation of the performance of methods for the elemental analysis of forensic glass by μ-XRF, ICP-MS, and LA-ICP-MS. Anal Bioanal Chem 405(16): 5393-5409.
- Muohi AW, Onyarib JM, Omondi JG, Mavuti KM (2003) Heavy metal in sediments from Makupa and port-Reitz Creek systems: Kenyan coast. Environment international 28: 639-647.
- Schwarz K, Pickett ST, Lathrop RG, Weathers KC, Pouyat RV (2012) The effects of the urban built environment on the spatial distribution of lead in residential soils. Environ Pollut 163: 32-39.
- Johnson DM, Hooper PR, Conrey RM (1999) XRF Analysis of Rocks and Minerals for Major and Trace Elements on a Single Low Dilution Li- Tetraborate Fused Bead. Advances in X-ray Analysis 41: 843-867.
- Shackley MS (2011) X‐Ray Fluorescence Spectrometry (XRF). The Encyclopedia of Archaeological Sciences p: 1-5.
- Oscar Díaz Rizo, Alina Gelen Rudnikas, Ruslán D, Lavin Pérez, Gustavo Arencibia Caraballo, et al. (2014) XRF analysis of sediments from Nuevitas Bay (Cuba): assessment of current heavy metal contamination. Nucleus 55: 11-14.
- Shefsky S (1997) Comparing Field Portable X-Ray Fluorescence (XRF) to laboratory analysis of heavy metals in soil. International Symposium of Field Screening Methods for Hazardous Wastes and Toxic Chemicals p: 1-6.
- Lei Y, Du X, Wang Y, Chen Q, Tang H, et al. (2018) Diatom succession dynamics controlled by multiple forces in a subtropical reservoir in southern China. Quaternary international 493(10): 227-244.
- Hu Y, Qi S, Wu C, Ke Y, Chen J, et al. (2012) Preliminary assessment of heavy metal contamination in surface water and sediments from Honghu Lake, East Central China. Frontiers of Earth Science 6(1): 39-47.
- Zhao H, Li X, Wang X, Tian D (2010) Grain size distribution of roaddeposited sediment and its contribution to heavy metal pollution in urban runoff in Beijing, China. J Hazard Mater 183(1-3): 203-210.
- Yu W, Zhang Y, Zhou W, Wang W, Tang R (2019) Urban expansion in Shenzhen since 1970s: A retrospect of change from a village to a megacity from the space. Physics and Chemistry of the Earth, Parts A/B/C.
- Lui TL, Chiu S (1994) A tale of two industries: the restructuring of Hong Kong’s garment-making and electronics industries, Environment and Planning A: Economy and Space 26(1): 53-70.
- Guangdong Provincial Coastal Zone and Coastal Resources Comprehensive Investigation Leading Group Office (1985). A paper collection for comprehensive survey of the Pearl River estuary coastal zone and tidal flat resources, China.
- Blackmore G (1998) An overview of trace metal pollution in the coastal waters of Hong Kong. Sci Total Environ 214: 21-48.
- Lui TL, Chiu S (1993) Industrial restructuring and labour-market adjustment under positive noninterventionism: the case of Hong Kong. Environment and Planning A: Economy and Space 25(1): 63-79.
- Wong C, Yeung H, Cheung R, Yung K, Wong MH (2000) Ecotoxicological assessment of persistent organic and heavy metal contamination in Hong Kong coastal sediment. Archives of Environmental Contamination and Toxicology 38(4): 486-493.
- Ong Che RG (1999) Concentration of 7 heavy metals in sediments and mangrove root samples from Mai Po, Hong Kong. Marine Pollution Bulletin 39(1-12): 269-279.
-
Caiying Zhong, Hong Su, Hongyu Yan , Shijun Jiang. Industrial Development of Hong Kong and its Environmental Impacts as Recorded by Sediment Pb Variation in Shenzhen Bay, South China. Ad Oceanogr & Marine Biol. 1(3): 2019. AOMB.MS.ID.000513.
-
Lead, Sediment, X-ray fluorescence (XRF), Grain size, Hong Kong, Shenzhen Bay, Heavy metal pollution, Marine environment, Volcanic eruption, Weathering, Erosion, Earth’s crust
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






