 Mini Review
Mini Review
Telomerase and the Brain: A Special Relationship
Gabriele Saretzki, The Ageing Biology Centre, Institute for Cell and Molecular Biosciences, Newcastle University, Campus for Ageing and Vitality, Newcastle upon Tyne, UK.
Received Date: February 18, 2019; Published Date: February 22, 2019
Abstract
While telomerase is best studied in its canonical function on telomere maintenance during cell division, various non-canonical functions beyond any telomere involvement and nuclear localisation of the protein part TERT (Telomerase Reverse Transcriptase) have been discovered in recent years. It currently emerges that the TERT protein seems to have a particular important function in the brain and neurons. While in most human somatic tissues the TERT component is downregulated while the RNA component TR/ TERC persists, in human brain it seems to be the opposite: the RNA component is downregulated early during development and TERT persists even in neurons from old brain. This mini-review gives a brief overview of the special relationship of telomerase in the brain which might be exploited in future therapies of neurodegenerative diseases.
Telomerase in Mammalian Cells
Telomerase is a reverse transcriptase that maintains telomeres in cells where it is active. For that function, 2 minimal components are required and sufficient in vitro [1]: the catalytic subunit TERT (Telomerase Reverse Transcriptase) and the RNA component TR/TERC which also contains the 11 nucleotide template region for the addition of TTAGGG hexanucleotides onto the 3’ G-rich telomeric overhang. This catalytic function of telomerase has been initially described by E. Blackburn and colleagues in unicellular protozoans [2]. However, it quickly emerged that most eukaryotic organisms use this ancient enzyme which, due to its composition, is a ribonucleoprotein, for maintaining linear chromosomal ends. Telomeres shorten during cell division predominantly due to the inability of the semiconservative DNA replication process which leaves an RNA primer at the very end which cannot be replaced by DNA [3]. This is also known as the “End Replication Problem” (ERP) [4]. In addition, oxidative stress can contribute to telomere shortening as well as telomeric DNA damage [5-7].
The catalytic function of telomerase has been well studied and described and plays a major role in dividing cells. However, telomere shortening, due to induction of cellular senescence or apoptosis, also acts as a tumour suppressor mechanism [8]. While most mouse tissues have telomerase activity present during adulthood in the majority of tissues and organs, most human somatic cells downregulate telomerase activity early during development [9- 11]. In contrast, germline cells, embryonic stem cells and most cancer cells have high, constitutive activity of telomerase which is a prerequisite for their immortality [12-14]. The majority of human somatic cells does not have telomerase activity. Exceptions are endothelial cells and lymphocytes such as T- and B-cells as well as adult stem cells that are both able to upregulate telomerase activity upon stimulation [15-17]. During differentiation of human embryonic stem cells in vitro telomerase activity and the expression of hTERT are downregulated quickly, while the hTR component is still detectable after differentiation [18]. This scenario most likely also occurs in vivo during development.
In general, it is thought, that the presence of the catalytic subunit TERT is the limiting factor for the presence of telomerase activity in cells, including human cells. This is the reason, why human somatic cells can be transfected by hTERT in order to generate telomerase activity and extend the lifespan of mortal cells and avoid senescence and apoptosis due to continuous telomere maintenance [19,20]. This is due to the fact that the RNA component is present in most human somatic cells and it had been speculated that it might fulfil other roles in there. In addition to its canonical function in telomere maintenance, over the past years it emerged that the catalytic subunit TERT has various non-canonical functions. This includes it’s shuttling to mitochondria where it protects cells from oxidative stress, DNA damage in mitochondrial and nuclear DNA as well as apoptosis [20-22]. TERT has a mitochondrial localisation signal and is exported from the nucleus upon phosphorylation in a Src-kinase dependent manner [23,24]. Moreover, TERT is able to complex with mitochondrial RNA’s in order to generate a reverse transcriptase function within the organelle [25], although it’s biological significance is not entirely clear. In addition, the non-canonical role of TERT has been shown to promote various properties of tumour cells such as migration, epithelial-mesenchymal transition (EMT), invasion and more [26].
Telomerase in Mouse and Human Brain Cells
Over the last years various groups have demonstrated a particular role of the TERT protein in brain and specifically in neurons [27-29]. Mouse and human microglia due to their macrophage-related origin display some telomerase activity and TERT protein [30,28]. However, contradicting data exists for rodent astrocytes, with some studies demonstrating initial but over time subsiding telomerase activity during in vitro culture [30] while other studies found a decrease of telomerase activity and TERT expression during in vitro differentiation of mouse neural stem cells into astrocytes [31]. Our group did not detect TERT protein in astrocytes of the human hippocampus and cultured mouse astrocytes [28]. However, we found TERT protein present even in brain neurons from old AD patients, while no telomerase activity is detectable in embryonic brains after post-conception week 10 when measured with a conventional TRAP assay at 30 cycles [32]. Our study demonstrated that instead of TERT, in human brain it seems to be rather the RNA subunit hTR that is downregulated. This could be the mechanism how telomerase activity is downregulated in the human brain in contrast to most other human tissues. Our results suggest a different mechanism of down-regulation of telomerase activity in human neurons compared to other human tissues (Figure 1).
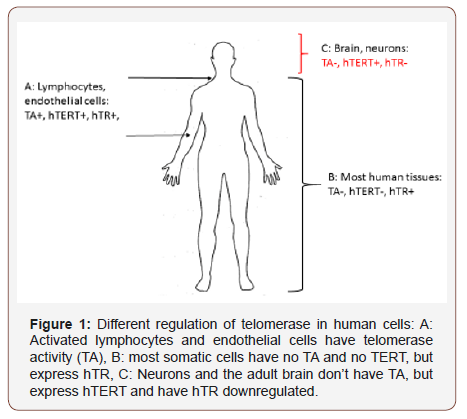
The situation is slightly different in mice where telomerase activity is still detectable in whole brain until early postnatal stages [33] and thus rodents have a slightly different telomerase biology and kinetics of downregulation of telomerase activity. However, in contrast to most other mouse and rodent tissues, adult rodent brain also does not have measurable telomerase activity unlike most other mouse organs [33]. Nevertheless, some aspects of telomerase biology in adult mouse brain seem to be rather similar to humans since the use of telomerase activators demonstrated beneficial effects in a model of Amytrophic Lateral Sclerosis (ALS) [34]. Our group has found that mTERT protein accumulates specifically in mouse brain mitochondria under conditions of dietary restriction (DR) and rapamycin treatment while it did not do the same in other tissues such as liver [35]. This observation confirmed again that TERT protein has a protective function specifically in brain mitochondria, not only under increased stress conditions as shown in cells and neurons previously [20,22,29], but also under beneficial physiological conditions. We also found that the decrease in oxidative stress due to decreased mTOR signalling was dependent on the presence of TERT protein [35,36]. Similarly, we had demonstrated previously in a neuronal model that the presence of TERT protected cells from oxidative stress and cellular damage caused by the expression of pathological tau [28].
Together, these results suggest that the presence of TERT might be particularly beneficial in the brain and neurons. This could be the underlying reason for the downregulation of the telomerase RNA component TR/TERC in brain while in other human somatic tissues it is TERT which is downregulated while hTR is present, but in both cases telomerase activity (TA) is absent. An overview about the differential regulation of telomerase including hTERT and hTR subunits in the human body is shown in Figure 1.
Importantly, the potentially beneficial effect of TERT in brain implies that increasing TERT levels using telomerase activators might be an attractive novel treatment option for therapies aiming to delay and ameliorate symptoms of neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) in the future.
Acknowledgemnet
None.
Conflict of Interest
No conflict of interest.
References
- Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, et al. (1997) Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet 17(4): 498-502.
- Blackburn EH, Greider CW, Henderson E, Lee MS, Shampay J, et al. (1989) Recognition and elongation of telomeres by telomerase. Genome 31(2): 553-560.
- Olovnikov AM (1995) The effect of the incomplete terminal repair of the linear double-stranded DNA molecule. Izv Akad Nauk Ser Biol (4): 501- 503.
- Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB (1992) Telomere end-replication problem and cell aging. J Mol Biol 225(4): 951-960.
- von Zglinicki T, Saretzki G, Döcke W, Lotze C (1995) Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res 220(1): 186-193.
- von Zglinicki T (2000) Role of oxidative stress in telomere length regulation and replicative senescence. Ann NY Acad Sci 908: 99-110.
- Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, et al. (2012) Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 3: 708.
- d’Adda di Fagagna F, Hande MP, Tong WM, Lansdorp PM, Wang ZQ, et al. (1999) Functions of poly (ADP-ribose) polymerase in controlling telomere length and chromosomal stability. Nat Genet 23(1): 76-80.
- Martín-Rivera L, Herrera E, Albar JP, Blasco MA (1998) Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc Natl Acad Sci U S A 95(18): 10471-10476.
- Ulaner GA, Giudice LC (1997) Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol Hum Reprod 3(9): 769-773.
- Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR (1998) Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res 58(18): 4168-4172.
- Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW (1996) Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 18(2): 173-179.
- Zeng X (2007) Human embryonic stem cells: mechanisms to escape replicative senescence? Stem Cell Rev 3(4): 270-279.
- Shay JW, Bacchetti S (1997) A survey of telomerase activity in human cancer. Eur J Cancer 33(5): 787-791.
- Liu K, Schoonmaker MM, Levine BL, June CH, Hodes RJ, et al. (1999) Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes. Proc Natl Acad Sci U S A 96(9): 5147-5152.
- Hiyama E, Hiyama K (2007) Telomere and telomerase in stem cells. Br J Cancer 96(7): 1020-1024.
- Hsiao R, Sharma HW, Ramakrishnan S, Keith E, Narayanan R (1997) Telomerase activity in normal human endothelial cells. Anticancer Res 17(2A): 827-832.
- Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, et al. (2008) Downregulation of multiple stress defence mechanisms during differentiation of human embryonic stem cells. Stem Cells 26(2): 455-465.
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, et al. (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279(5349): 349-352.
- Ahmed S, Passos JF, Birket MJ, T Beckmann, S Brings, et al. (2008) Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Science 17: 1046- 1053.
- Haendeler J, Dröse S, Büchner N, Jakob S, Altschmied J, et al. (2009) Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler Thromb Vasc Biol 29(6): 929-935.
- Singhapol C, Pal D, Czapiewski R, Porika M, Nelson G, et al. (2013) Mitochondrial telomerase protects cancer cells from nuclear DNA damage and apoptosis. PLoS One 8(1): e52989.
- Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B (2004) Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell 3(6): 399-411.
- Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S (2003) Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol Cell Biol 23(13): 4598-4610.
- Sharma NK, Reyes A, Green P, Caron MJ, Bonini MG, et al. (2012) Human telomerase acts as a hTR-independent reverse transcriptase in mitochondria. Nucleic Acids Res 40: 712-725.
- Saretzki G (2014) Extra-telomeric Functions of Human Telomerase: Cancer, Mitochondria and Oxidative Stress. Curr Pharm Des 20(41): 6386-6403.
- Iannilli F, Zalfa F, Gartner A, Bagni C, Dotti CG (2013) Cytoplasmic TERT associates to RNA granules in fully mature neurons: role in the translational control of the cell cycle inhibitor p15INK4B. PLoS One 8(6): e66602.
- Spilsbury A, Miwa S, Attems J, Saretzki G (2015) The role of telomerase protein TERT in Alzheimer’s disease and in tau-related pathology in vitro. J of Neuroscience 35(4):1659-1674.
- Eitan E, Braverman C, Tichon A, Gitler D, Hutchison ER, et al. (2016) Excitotoxic and Radiation Stress Increase TERT Levels in the Mitochondria and Cytosol of Cerebellar Purkinje Neurons. Cerebellum 15(4): 509-517.
- Flanary BE, Streit WJ (2004) Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia 45(1): 75-88.
- Miura T, Katakura Y, Yamamoto K, Uehara N, Tsuchiya T, et al. (2001) Neural stem cells lose telomerase activity upon differentiating into astrocytes. Cytotechnology 36(1-3): 137-144.
- Ishaq A, Hanson PS, Morris CM, Saretzki G (2016) Telomerase Activity is Downregulated Early During Human Brain Development. Genes (Basel) 7(6): E27.
- Klapper W, Shin T, Mattson MP (2001) Differential regulation of telomerase activity and TERT expression during brain development in mice. J Neurosci Res 64(3): 252-260.
- Eitan E, Tichon A, Gazit A, Gitler D, Slavin S, et al. (2012) Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol Med 4(4): 313-329.
- Miwa S, Czapiewski R, Wan T, Bell A, Hill KN, et al. (2016) Decreased mTOR signalling reduces mitochondrial ROS in brain via accumulation of the telomerase protein TERT within mitochondria. Aging (Albany NY) 8(10): 2551-2567.
- Miwa S, Saretzki G (2017) Telomerase and mTOR in the brain: the mitochondria connection. Neural Regen Res 12(3): 358-361.
-
Gabriele Saretzki. Telomerase and the Brain: A Special Relationship. Arch Neurol & Neurosci. 2(4): 2019. ANN.MS.ID.000545.
-
Telomerase, Brain, Ageing Biology, Neurons, DNA damage, Apoptosis, Tumour suppressor, cancer cells, Epithelial-mesenchymal transition, Mouse, Human somatic cells, Migration, Neurodegenerative diseases, Alzheimer’s disease (AD), Parkinson’s disease (PD)
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






