 Research Article
Research Article
Neural Conductional Impairment in the Brainstem Auditory Pathway in term Infants with Perinatal Problems
James Ken Jiang1 Cui Wong2 and Ze Dong Jiang2*
1School of Physiology, Pharmacology and Neuroscience, University of Bristol, Bristol, UK,
2Division of Neonatology, Children’s Hospital of Fudan University, Shanghai, China
Ze Dong Jiang, Division of Neonatology, Children’s Hospital of Fudan University, Shanghai, China.
Received Date: November 01, 2021; Published Date: November 23, 2021
Abstract
Term infants with perinatal problems, i.e., high risk term infants, often suffer certain degree of brain damage. We examined evoked responses in the brainstem auditory pathway in high risk term infants in a tertiary hospital. The data were compared with those in normal term controls to identify any difference. To minimize the influence of threshold variation on wave latencies of the evoked response, all data were analyzed at 40 dB or slightly higher above individual threshold. Compared with the normal controls, the high risk infants showed slightly increase in wave I latency. However, the latencies of waves III and V in the high risk infants were both increased significantly at all repetition rates of clicks used. The I-V interpeak interval was increased significantly at all click rates. Although the I-III interval in the high risk infants tended to be increased, no significant difference from the normal controls was found at any click rates. Nevertheless, the III-V interval in the high risk infants was significantly increased at all click rates. These results suggest neural conductional impairment in the brainstem auditory pathway in term infants with perinatal problems.
Introduction
Most full-term neonates are born with no major perinatal
problems. The others, however, have various perinatal conditions
or problems. A most prominent perinatal problem that significantly
damage the immature brain is perinatal asphyxia. With the
improvement in perinatal care, the incidence of perinatal asphyxia
has reduced significantly. However, there are still various perinatal
problems that may directly or indirectly damage the immature
brain, leading to various degree of neurological impairment and/
or developmental deficits. The brainstem auditory evoked response
(BAER), reflecting functional integrity of the brainstem auditory
pathway, can help detect auditory impairment and brain damage
[1,2]. The BAER test is particularly suitable for neonates who have
perinatal problems that may affect the auditory pathway [2,3]. A
large body of BAER studies have shown that the neonatal brainstem
auditory pathway is susceptible to some unfavorable perinatal
conditions or problems, e.g., asphyxia, hyperbilirubinemia, and
meningitis [2-6]. Previous reports on the BAER were mostly in
preterm infants, whereas the reports in term infants were relatively
fewer.
An improved understanding of whether term neonates with
perinatal problems, i.e., high risk term infants, are associated with
brainstem auditory impairment is important for management of
these infants. In this reported study, we recorded and analyzed
BAER in high-risk term infants at a neonatal intensive care unit in a
tertiary hospital. Perinatal asphyxia is a well-known high-risk factor
for the immature brain, including the brainstem auditory pathway
[1-3,6]. To exclude the known effect and focus on other perinatal
problems or conditions that may affect the brainstem auditory
pathway, we exclude any infants who had perinatal asphyxia. The
data were compared with those in normal term infants to identify any difference. The BAER was recorded and analysed with click
stimuli of different repetition rates. A detailed analysis of BAER
wave latencies and interpeak intervals was conducted to focus on
neural conduction in the brainstem auditory pathway. To minimize
the influence of threshold variation on BAER wave latencies, all data
were analysed at 40 dB or slightly higher above BAER threshold of
individual infants.
Patients and Methods
The study group was comprised of 47 term neonates who had
various perinatal conditions and/or problems, except asphyxia.
They were recruited from the neonatal intensive care unit of the
Children’s Hospital, Fudan University. The perinatal conditions
and/or problems included meconium aspiration syndrome,
hyperbilirubinemia, hypotension, sepsis, metabolic acidosis,
hypoglycaemia, etc. Their gestational age was 37-42 (39.6 ± 1.4)
and birthweight 2505-4,815g (3,404 ± 565g). Apgar scores were
7-10 at both 1- and 5-minutes. The normal control group was
comprised of 44 healthy term neonates. Their gestational age was
37-42 (39.2 ± 1.2) and birthweight 2534-4,846g (3,436 ± 4725g).
Neither gestational age nor birthweight differed statistically
between the study and control groups.
All infants were studied at 3-6 days after birth. At the time of
BAER testing, the postconceptional age was 40.2 ± 1.3 and 39.6 ±
1.4, respectively, for the study and control groups, which did not
differ significantly. Before the test, parental approval was obtained
for all infants. The study protocols were approved by the Ethics
Committee of the Children’s Hospital. Only the left ear was tested
for consistency and reducing recording and analysing time. The
BAER was recorded and analysed using a Spirit 2000 Portable
Evoked Potential System (Nicolet Biomedical Inc. USA). Sweep
duration was 12 ms. Prior to BAER recording the auditory meatus
was inspected and cleaned of any vernix or wax. The infant lay
supine in the cot. Before electrode placement, the skin on sites
was prepared to reduce interelectrode impedances to 5 kΩ and
less. Gold-plated disk electrodes were placed, respectively, at the
middle forehead (+), the ipsilateral earlobe (-) and the contralateral
earlobe (ground). Recording BAER commenced shortly after the
subjects fell asleep naturally, often after a feed, without using any
sedatives. Rarefaction clicks of 100 μsec duration were delivered
to the left ear through a TDH 39 earphone. All subjects were tested
at a click intensity 60 dB nHL. For those who had a BAER threshold
>20 dB nHL, higher intensities were used to obtain BAER data at an
intensity level 40 dB or slightly higher above the threshold of each
subject. This allowed BAER components to be clearly identified and
reliably analysed. Two runs were made for each recording condition
for reproducibility. Each run included averaged brain responses to
2,048 clicks. The clicks were presented at the sequence of 21, 51
and 91/s in the first run, and at a reversed sequence in the second
run.
Prior to inputting to the averager brain responses to the
clicks were amplified and bandpass filtered at 100-3000 Hz. An
automatic artefact rejection was used to reduce the inclusion of
high-amplitude muscular activity in the averaged responses. Both
the ongoing filtered EEG and the running averaged BAER were
monitored while averaging. Whenever there were excessive muscle
artefacts on the monitoring screen the sampling was manually
discontinued to avoid inclusion of the artefacts. Sweep duration
was 12 ms. BAER data collected with the click intensity of 60 dB
nHL were used for detailed analysis. In the high risk infants who
had a BAER threshold between 25 and 40 dB nHL (n = 6), the BAER
was analysed with the data collected with clicks at 70-90 dB nHL.
This will allowed the data in all infants to be analysed at a hearing
level ≥ 40 dB or slightly higher above the threshold of each infants.
The measurements of two replicated BAER recordings to each
stimulus condition were averaged for further analysis. The mean
and standard deviation of each BAER variable were compared
between the study and control groups using a Student’s t test. A
2-tailed value of p <0.05 was considered statistically significant.
Results
For all infants, BAER waves I, III and V were reliably identified
at all click rates used. This was mainly benefited from the fact that
all data were analysed at a hearing level ≥40 dB above the threshold
of each neonate. The measurements of BAER wave latencies and
interpeak intervals at various click rates are presented in Figs.
1-7. As the click rate was increased, all BAER wave latencies and
interpeak intervals in both the study and control groups. In the
study group, the latencies of waves I, III and V were all correlated
positively and significantly with click rate (r = 0.432, 0.541 and
0.687, all P <0.01). The I-III, III-V and I-V intervals were also
correlated positively and significantly with the rate (r = 0.412, 0.635
and 0.616, all P < 0.01). This is also for the III-V/I-III interval ratio (r
= 0.425, P <0.01). These correlations between BAER variables and
click rate in the study group were generally similar to those in the
control group, with only small differences.
Compared with the control group, the study group showed
a general increase in most BAER wave latencies and interpeak
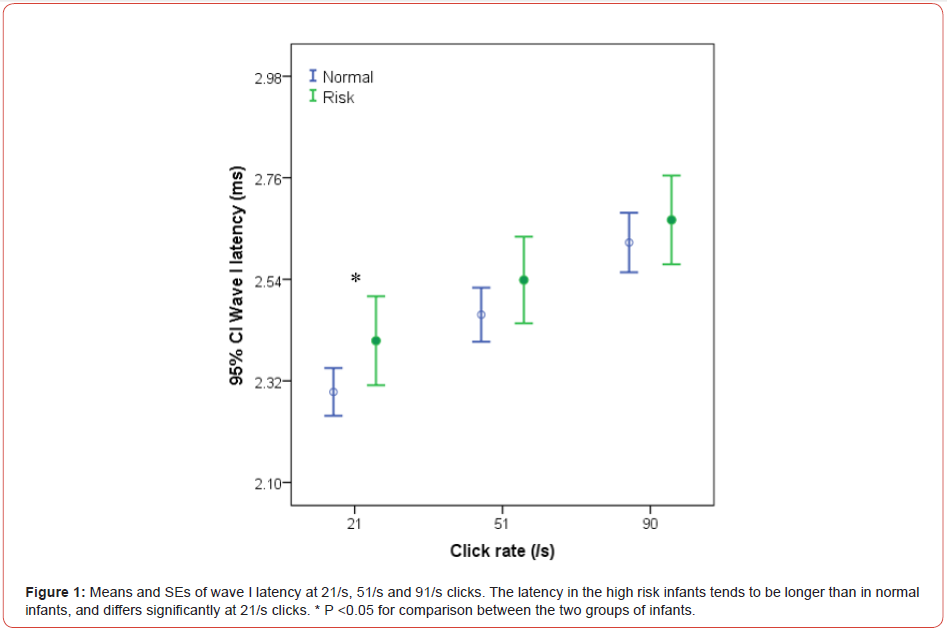
intervals (Figures 1-7). Wave I latency in the study group tended
to be longer than in the control group at all 21/s, 51/s and 91/s,
but only differed significantly at 21/s clicks (P <0.05) (Figure 1).
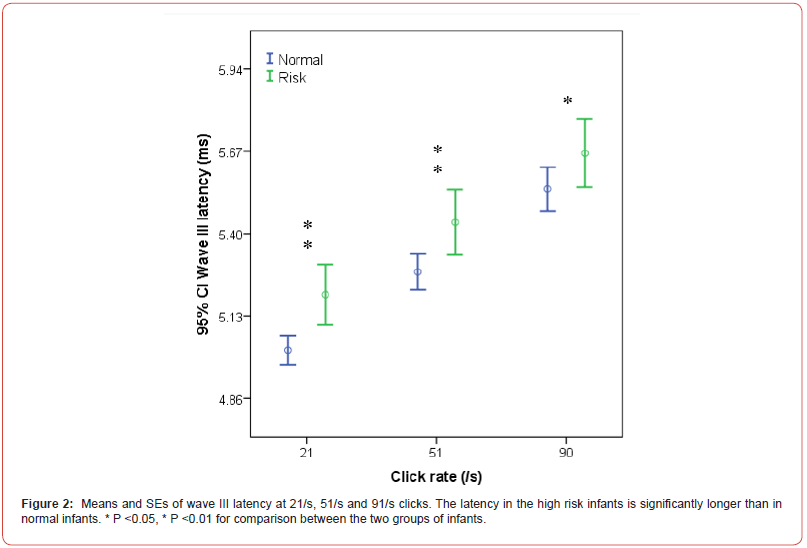
However, wave III latency in the study group was significantly longer
than in the control group at all click rates (P <0.05-0.01) (Figure 2).
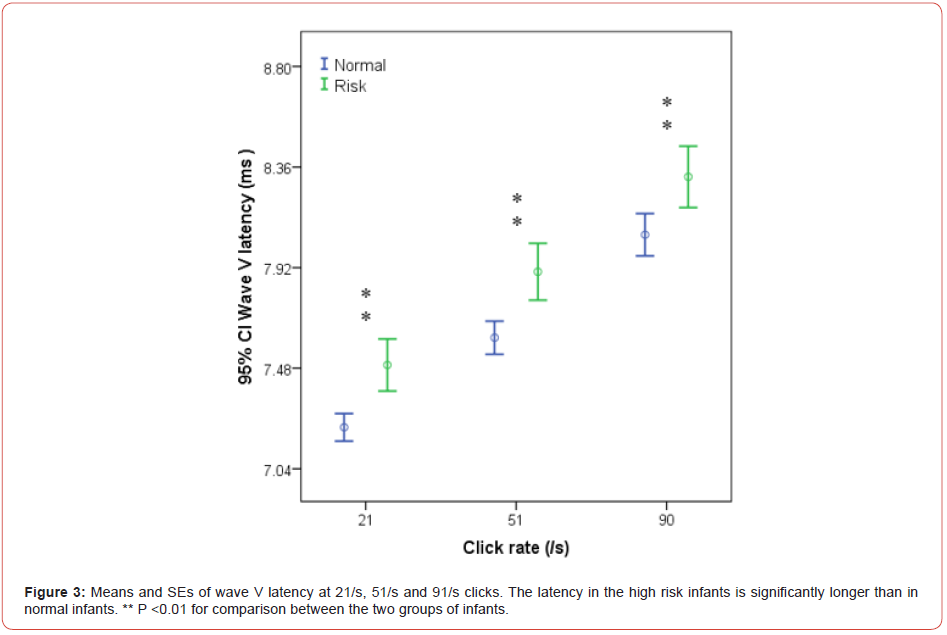
Similarly, wave V latency in the study group was significantly longer
than in the control group at all click rates (all P <0.01) (Figure 3).
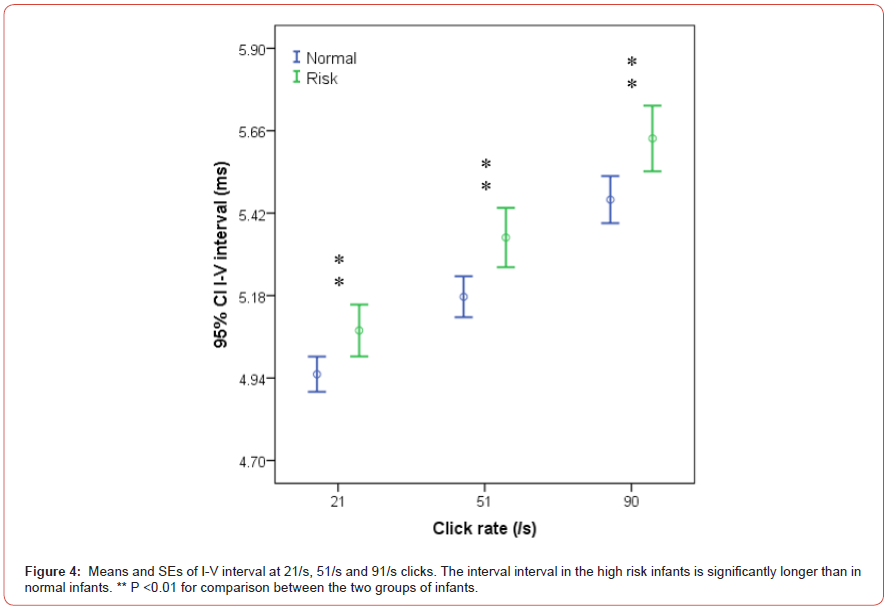
The I-V interpeak interval in the study group was significantly
longer than in the control group at all click rates (all P <0.01)
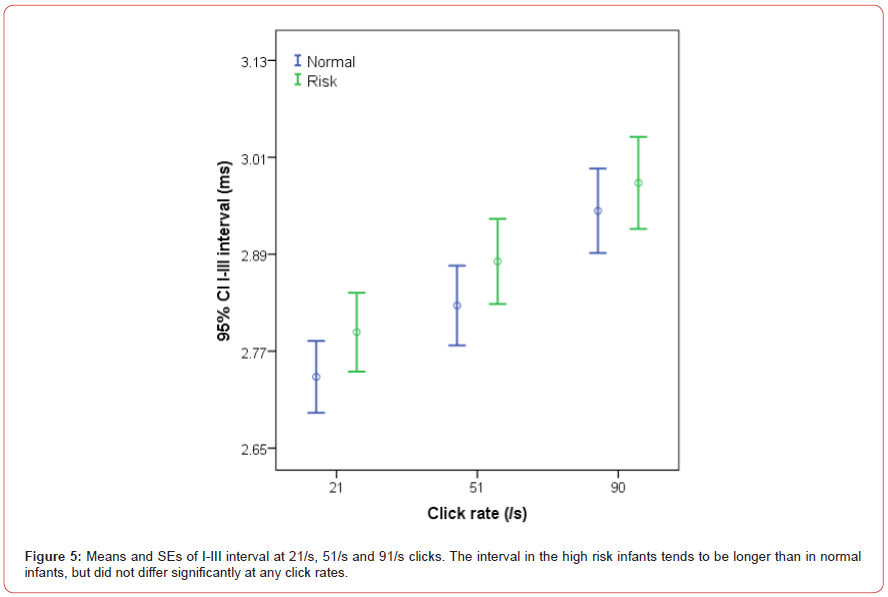
(Figure 4). The I-III interval tended to be longer than in the study
group, but the difference between the two groups did not reach
statistical significance at any click rates (Figure 5). However, the
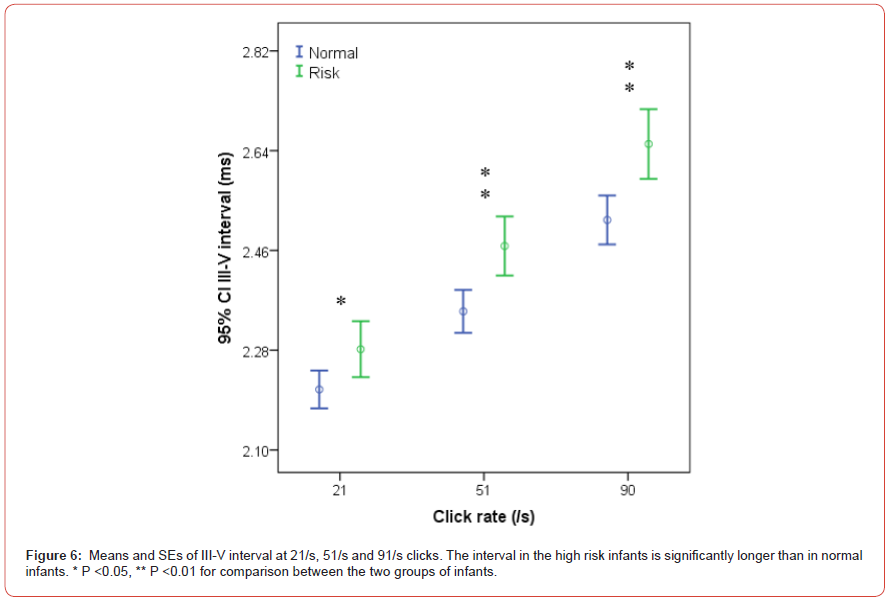
III-V interval in the study group was significantly longer than in the
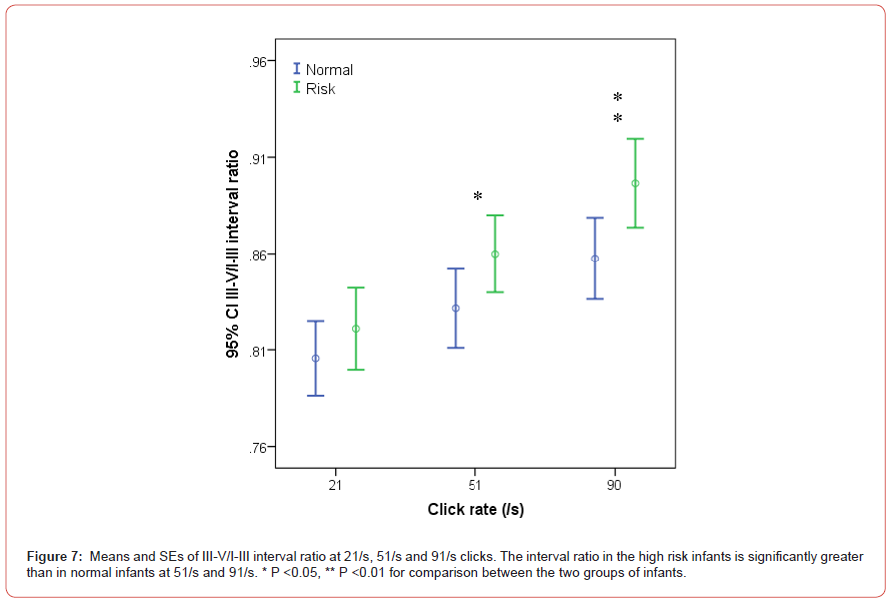
control group at all click rates (all P <0.01) (Figure 6). The III-V/IIII
interval ratio in the study was slightly longer than in the control
group, but significantly longer than at both 51/s and 91/s clicks (P
<0.05 and 0,01) (Figure 7). Clearly, the difference between the two
groups was increased with click rates increase.
Among perinatal problems, asphyxia is the most extensively
studied by previous investigators using the BAER [1,3,6]. It is now
evident that perinatal asphyxia often results in BAER abnormalities,
including increase in wave latencies and interpeak intervals,
which suggests functional impairment of the auditory pathway. By
comparison, it is relatively less clear if other perinatal problems
result in BAER abnormalities in term infants at term age. In the
present study, we found major BAER abnormalities in the high
risk term infants within one week after birth, suggesting neural
conductional impairment in the brainstem auditory pathway. In the
BAER, wave I is generated exclusively by the eighth nerve, located
in the peripheral part of the brainstem auditory pathway. Wave III
is mainly generated by the neurons in the cochlear nucleus, located
in the dorsal or more peripheral regions of the auditory brainstem.
Wave V is generated by the auditory neurons in the nuclei of the
lateral lemniscus and/or inferior colliculus, located in the rostral or
more central regions of the brainstem [1,7,8]. In the present study,
although wave I latency was only slightly increased, the latencies
of BAER waves III and, particularly, V were in our high risk infants
significantly increased. Generally, the latencies of later BAER
waves, i.e., waves with longer latencies, were increased more than
the latencies of earlier waves, i.e., waves with shorter latencies.
The same is true at all click rates. This finding suggests that the
more central BAER components are increased more than the more
peripheral components in the high risk infants, which is confirmed
by the findings of BAER interpeak intervals. The I-V interval is the
most widely used BAER variable to assess neural conduction along
the brainstem auditory pathway. The significant increase in this
interval in our high risk infants at all click rates used is indicative
of impaired neural conduction along the pathway. Of the two subcomponents
of the I-V interval, the first or earlier sub-component
I-III interval was only slightly increased, while the second or later
sub-component III-V interval was significantly increased at all click
rates. Clearly, the significant increase in the I-V interval (and wave
V latency) in the high risk infant is produced mainly by the increase
in the III-V interval. These results indicate that high risk term
infants are associated with impaired neural conduction along the
brainstem auditory pathway. The perinatal problems or conditions
other than asphyxia affect the brainstem auditory pathway and
damage neural conduction of the pathway. The damage occurs
mainly at more central part of the pathway.
Asphyxia has long been recognized to damage the brainstem
auditory pathway [1,3,9,12]. In addition, there are many other
perinatal problems that could damage the immature brain, e.g.,
sepsis, hyperbilirubinemia [13-21]. In the present study, because of
the limited number in each of the problems, it is difficult to identify
which of these play(s) a major role in the BAER abnormalities.
Limited information is available in the literature regarding BAER
findings in any specific perinatal problem or complication. It is
likely that the BAER abnormalities found in our high risk infants are
the results of collective adverse effects by more than one perinatal
problems or conditions. It is important to identify any perinatal problems, in addition to the known perinatal asphyxia, that damage
the brainstem auditory pathways. To do so a substantial number
of subjects are required so that each risk factor has a sufficient
number of subjects for robust statistical analysis. Nevertheless,
the sample size of the present study was relatively small and the
number of subjects for each perinatal condition is not sufficient for
robust, reliable statistical analysis. With continuing to recruit new
subjects, we hope that in time our data will be sufficient for reliably
identify the major high risk factor(s) that damage the brainstem
auditory pathway.
References
- Jiang ZD (2015) Evoked potentials in pediatric brainstem lesions. In: Galloway G, editor. Clinical Neurophysiology in Pediatrics: A Practical Approach to Neurodiagnostic Testing and Management. New York: Demos Medical Publishing, LLC: 187-213.
- Hall III JW (2007) ABR: Pediatric clinical application and populations. In: Hall III JW, editor. New Handbook of Auditory Evoked Responses. Boston: Pearson Education 13-365.
- Wilkinson AR, Jiang ZD (2006) Brainstem auditory evoked response in neonatal neurology. Semin Fet Neonatol Med 11: 444-451.
- Majnemer A, Rosenblatt B (1996) Evoked potentials as predictors of outcome in neonatal intensive care unit survivors: review of the literature. Pediatr Neurol 14: 189-195.
- Majnemer A, Rosenblatt B (2000) Prediction of outcome at school age in neonatal intensive care unit graduates using neonatal neurologic tools. J Child Neurol 15: 645-651.
- Jiang ZD (2013) Brainstem auditory evoked response in neonatal brain damage. Cur Trend Neurol 7: 1-14.
- Moore JK (1987) The human auditory brain stem as a generator of auditory evoked potentials. Hear Res 29: 33-43.
- Moore JK, Perazzo LM, Braun A (1995) Time course of axonal myelination in the human brainstem auditory pathway. Hear Res 87: 21-31.
- Newton V (2001) Adverse perinatal conditions and the inner ear. Semin Neonatol 6: 543-551.
- Jiang ZD (2008) Brainstem electrophysiological changes after perinatal hypoxia ischemia. In: Hämäläinen E. editor, New Trends in Brain Hypoxia Ischemia Research. New York: Nova Science Publishers, Inc., p. 203-220.
- Jiang ZD (2008) Brainstem electrophysiological changes after perinatal hypoxia ischemia. In: Hämäläinen E. editor, New Trends in Brain Hypoxia Ischemia Research. New York: Nova Science Publishers, Inc, ISA, pp. 203-220.
- Jiang ZD, Xu X, Yin R, Shao XM, Wilkinson AR (2004) Differential changes in peripheral and central components of the brainstem auditory evoked potentials during the neonatal period in term infants after perinatal hypoxia-ischaemia. Ann Otol Rhinol Laryngol 113: 571-576.
- Adams-Chapman I, Stoll BJ (2006) Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis 19: 290-297.
- Akinpelu OV, Waissbluth S, Daniel SJ (2013) Auditory risk of hyperbilirubinemia in term newborns: a systematic review. Int J Pediatr Otorhinolaryngol 77: 898-905.
- Burns CM, Rutherford MA, Boardman JP, Cowan FM (2008) Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics 122: 65-74.
- Johnson L, Bhutani VK (2011) The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin Perinatol 35: 101-113.
- Mallard C, Wang X (2012) Infection-induced vulnerability of perinatal brain injury. Neurol Res Int 2012: 102153.
- Montassir H, Maegaki Y, Ogura K, Kurozawa Y, Nagata I, et al. (2009) Associated factors in neonatal hypoglycemic brain injury. Brain Dev 31: 649-656.
- Shapiro SM (2010) Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med 15: 157-163.
- Tam EW, Haeusslein LA, Bonifacio SL, Glass HC, Rogers EE, et al. (2012) Hypoglycemia is associated with increased risk for brain injury and adverse neurodevelopmental outcome in neonates at risk for encephalopathy. J Pediatr 161: 88-93.
- Yalnizoglu D, Haliloglu G, Turanli G, Cila A, Topcu M (2007) Neurologic outcome in patients with MRI pattern of damage typical for neonatal hypoglycemia. Brain Dev 29: 285-292.
-
James Ken Jiang, Cui Wong, Ze Dong Jiang. Neural Conductional Impairment in the Brainstem Auditory Pathway in term Infants with Perinatal Problems. Arch Neurol & Neurosci. 11(5): 2021. ANN.MS.ID.000773.
-
Neural Conductional, Brainstem Auditory, Neonatology, Immature Brain, Neurological Impairment, Asphyxia, Hyperbilirubinemia, Asphyxia, Hypoglycaemia, Neurons.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






