 Research Article
Research Article
Stroke as a Very Rare Complication of Multile Bee Sting: A Case Report
Dmytro Filimonov1,3, Stanislav Yevtushenko3, Alexander Yeresko2, Anna Fedorova1, Margarita Belotserkovskaya1 and Nadezhda Trubnikova1
1Department of Experimental Surgery, V.K. Gusak Institute of Urgent and Reconstructive Surgery, Ukraine
2L.M. Litvinenko Institute of Physical Organic Chemistry and Coal Chemistry, Ukraine
3M. Gorky Donetsk National Medical University, Ukraine
Dmytro Filimonov, PhD MD, V.K. Gusak Institute of Urgent and Reconstructive Surgery, Department of Experimental Surgery, Donetsk, Ukraine.
Received Date: February 18, 2020; Published Date: March 05, 2020
Abstract
Background: According to recent studies, thyroid hormones may have various effects on stroke severity, course and outcome, but underlying mechanisms of this association are still unclear.
Objective: The aim was to determine the relationship of thyroid hormones during stroke onset with stroke severity and outcome in clinical study.
Methods: In this study 168 adult patients with acute ischemic stroke were enrolled. Concentrations of free T3 (fT3), free T4 (fT4), TSH and basic stroke risk factors were assessed during 24h from symptoms onset. Neurological deficit was assessed by Scandinavian Stroke Scale (SSS). Disabling deficit was defined as mRs score ≥3 at 6 months after stroke.
Results: ANOVA showed that SSS scores were significantly higher in patients with fT3 level in 4th quartile (≥5.35 pmol/l) compared to 2-3rd quartile (SSS median 48 vs. 37, p=0,0481) and especially to 1st quartile (≤3,4050 pmol/l, SSS median 48 vs. 30, p=0,0018). In patients without prior stroke (n=124) baseline SSS-score was independently affected by fT3 (corrected R2 = 0.49, p < 0.0001). According to ROC-analysis, fT3 level <4,44 pmol/l was a predictor of disabling deficit (AUC = 0.727, specificity - 96.4%, sensitivity - 66.8%, p = 0.003). Univariate analysis showed association between poor outcome and low fT3, in multiple regression this association became insignificant only after correction for baseline SSS-score, but no other stroke risk factors
Conclusion: The study showed that a low serum free triiodothyronine level during stroke onset negatively affects the stroke severity in firsttime stroke patients and may be predictor of its unfavorable outcome. There was a trend for association between low free triiodothyronine and unfavorable stroke outcome after 6 months. Beneficial effects of additional fT3 supplement during stroke should be assessed in future studies.
Keywords: Ischemic stroke; Outcome; Thyroid hormones; Triiodothyronine
Abbreviations: AIS: Acute Ischemic Stroke; fT3: Free Triiodothyronine; fT4: Free Thyroxine; TSH: Thyroid-Stimulating Hormone; CRP: C-Reactive Protein; CI: Confidential Interval; ATP: Adenosine Diphosphate; SSS: Scandinavian Stroke Scale; mRs: Modified Rankin Scale
Introduction
Currently, in most countries, stroke is one of the leading causes of death and disability [1]. Despite certain advances in the treatment and prevention of cerebrovascular diseases, stroke remains in second place in the world among the diseases leading to death. According to numerous clinical studies, the most effective treatment for acute ischemic stroke is the restoration of cerebral blood flow by reperfusion methods, such as intravenous or selective thrombolysis, or mechanical thrombectomy [2,3]. However, reperfusion therapy is possible only within a relatively narrow therapeutic window [4]. In cases where thrombolysis or thrombectomy is not indicated or there are no opportunities for its implementation, modern approaches to patient management during the acute period of stroke include both secondary prevention of cerebrovascular disease and an attempt to reduce the severity of neurological deficit by protecting ischemic (but potentially viable) brain tissue in penumbra zone [5]. Nevertheless, despite the diversity of neuroprotective drugs, different in their mechanism of action and effective in preclinical studies, none of them has enough clinical efficacy. In this regard, the search for new approaches of neuroprotection remains one of the most important tasks of modern neuropharmacology [5].
The survival of the brain tissue under ischemia depends on the intensity of metabolism, oxygen demand, as well as the ability to maintain the redox potential and support the synthesis of highenergy compounds (ATP, etc.). The mechanism of action of most neuroprotectors is based on the effects on these processes [6,7]. Over the past decades, special attention had been paid to the neuroprotective properties of endogenous molecules such as VEGF, erythropoietin, brain neurotrophic factor, etc. It is known that triiodothyronine, an active form of the thyroid hormone thyroxin, separates tissue respiration and oxidative phosphorylation. This process leads to disruption of the Krebs cycle, reduced ATP production, hyperthermia, and has a potentially negative effect in acute cerebral ischemia [8]. On the other hand, it is known that triiodothyronine has several neuroprotective effects - it contributes to the capture of neurotoxic glutamate by astrocytes, stimulation of the Na + / K + membrane channels in neurons, the restoration of intracellular pH [9]. Thus, selective therapeutic effect on thyroid metabolism (stimulation or inhibition of the function of thyroid hormones) may be promising potential target for new approaches in the treatment of stroke.
In recent years, more publications appeared in the literature about the possible effect of thyroid hormones on the risk of development, severity and outcome of acute ischemic stroke. Nevertheless, the results of the published works are rather contradictory [10]. Finally, the nature of the influence of hyperor hypothyroidism on the course and outcome of a stroke is still unclear.
Aims
Aim of this study was to determine the relationship between markers of thyroid function and the severity of neurological and functional deficit in acute ischemic stroke.
Materials and Methods
This study was conducted at the single clinical and research center – V.K. Gusak Institute of Urgent and Reparative Surgery (Donetsk). 168 patients (women – 71, men – 97) aged 42 to 78 years with acute ischemic heterogeneous stroke were enrolled in this study. Patients with verified autoimmune thyroiditis or a malignancy were excluded from the study. Within 24 hours from stroke onset basic risk stroke factors were analyzed. Serum free triiodothyronine (fT3), free thyroxine (fT4) and the thyroid stimulator hormone (TSH) were determined using ELISA method (CHEMWELL EIA-analyzer with DRG-international assay kits). Blood sampling was performed during 24 hours from stroke onset. Neurological deficit was assessed using Scandinavian Stroke Scale (SSS). Poor stroke outcome was assumed as 3 or more points on the modified Rankin Scale (mRs) after 6 months from stroke onset. Thyroid hormones and TSH levels below 25 and above 75 percentiles were assumed as “low” and “high”, respectively.
Statistical analysis
Statistical analysis was performed using MedCalc v14 software. Continuous data with non-normal distribution is presented as median and 95% CI. For analysis of variation of neurological deficit ANOVA method was used and patients were divided to subgroups according to T3 levels: “hypothyroid” (T3 below 25-percentile), “euthyroid” (T3 in 25-75 percentile), “hyperthyroid” (T3 above 75 percentile). For determining impact of thyroid hormones on stroke outcome using logistic regression patient were dichotomized in subgroups with good outcome (mRs 0-2) and poor outcome (mRs 3-6).
Results
Strokes in the carotid territory were the most frequent (79% of all patients), with the atherothrombotic subtype being the most common (64%). The leading basic risk factors were arterial hypertension (65%), smoking (42%), coronary heart disease (29%), atrial fibrillation (27%). 49 patients (28%) had previously history of TIA or ischemic stroke, 119 patients had the ischemic stroke developed for the first time. Demographic characteristics of the examined patients are presented in Table 1.
Table 1:Facial functions of the patients before the treatment.
According to the laboratory reference indicators, 112 patients had thyroid hormones levels in reference range, 36 patients had laboratory hypothyroidism, and 20 patients had laboratory hyperthyroidism. The distribution of thyroid hormones levels is presented in Table 2.
Correlation analysis using the Spearman rank correlation method revealed a positive statistically significant relationship between the level of free triiodothyronine and the severity of neurological deficit according to the SSS scale. A statistically significant relationship was also found between the level of free thyroxine and the presence of atrial fibrillation. Among other factors, an inverse correlation was observed between the level of C-reactive protein and the severity of neurological deficit according to the SSS scale (R = -0.397, p = 0.0004). No other significant correlation relationships were found between thyroid hormones, severity of neurological deficiency, and the presence of basic risk factors for stroke. The results of the correlation analysis are presented in Table 3.
Table 2:Distribution of thyroid hormones levels.

Table 3:Correlation relationships between thyroid function indicators, severity of neurological deficit, and stroke risk factors.

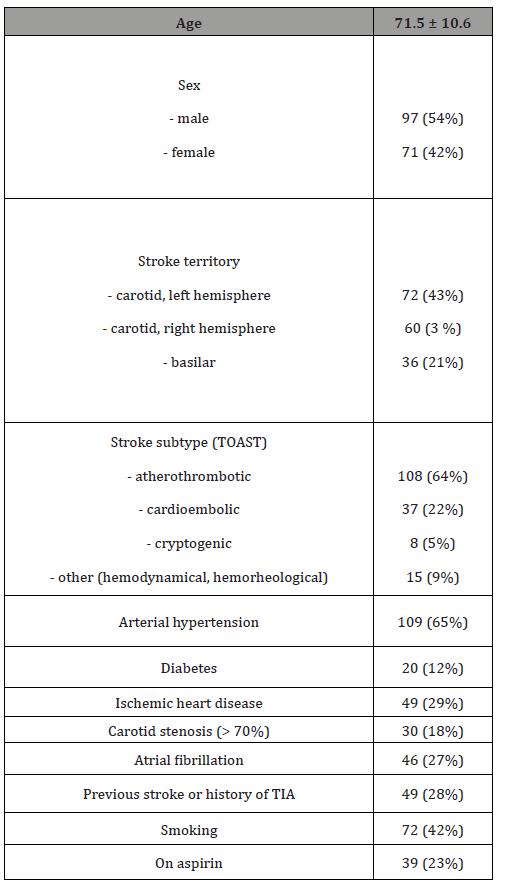
The ANOVA test showed that patients with fT3 level in the IV quartile (≥ 5.35 pmol/L; 95% CI 5.01 - 5.61) had less severe stroke (bigger SSS scores) compared to patients with fT3 level in II - III quartile (SSS median is 48 points in Q4 vs 37 points in Q2-3, p = 0,0481), and significantly especially less severe stroke compared to patients whose T3 level was in the first quartile (≤ 3.4050 pmol/l, SSS median 48 points in Q4 vs 30 points in Q1, p = 0.0018) . The results of the ANOVA test are presented in Figure 1.
The result of ANOVA test suggests that low triiodothyronine levels are associated with a more severe neurological deficit, while high levels may have potential neuroprotective effects. There were no statistically significant variations of SSS score in subgroups of patients according to fT4 or TSH levels.
After 6 month 120 patients were classified as having stroke with poor outcome and 48 patients had stroke with favorable outcome. Comparative analysis showed that patient with poor outcome were older, had significantly lower fT3 level, higher CRP levels and lower freeT3 to freeT4 ratio (Table 4).
Free T3 – free triiodothyronine, Free T4 – free thyroxine, TSH – thyroid-stimulating hormone. To clarify the relationship between the severity of neurological deficit and the level of free triiodothyronine in serum, a regression analysis was performed. Univariate regression analysis showed that in patients with cardioembolic stroke, fT4 favorably influenced the severity of the stroke according to the SSS scale (R2 = 0.75, p = 0.0005), but this association diminished after adjustment for other stroke risk factors (age, hypertension, carotid stenosis, glucose level, C-reactive protein, SSS score).
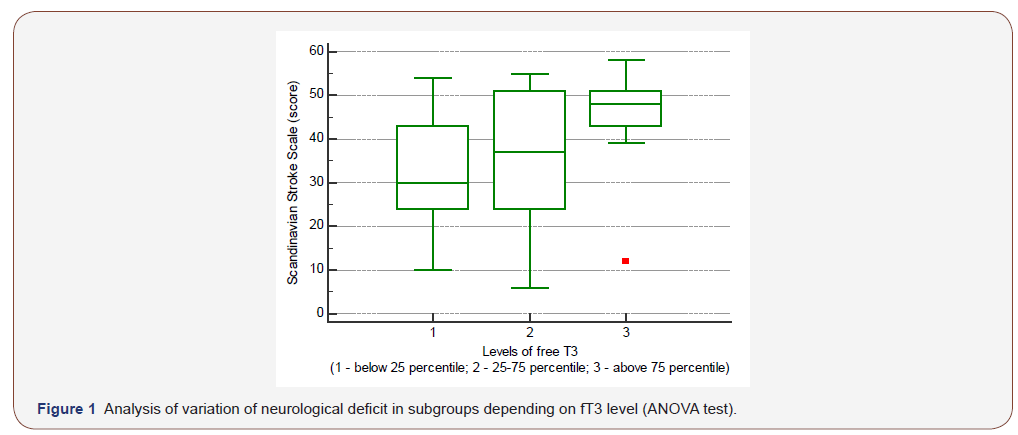
Table 4:Thyroid hormones, CRP levels and age of patient with different stroke outcomes.

Univariant regression showed no relationship between the severity of neurological deficit and the level of triiodothyronine. However, after exclusion of patients with previous stroke, a regression dependence of the severity of neurological deficit on the level of free triiodothyronine was detected (F = 15.7920, p = 0,0002, corrected R2 = 0,44). There were no significant differences in thyroid hormones levels, CRP and SSS-scores in patients with firsttime stroke and patients with history of stroke or TIA. To confirm the independent effect of the fT3 level on the severity of stroke and exclude the influence of co-factors, a multivariate regression analysis was performed. The regression model included basic risk stroke factors (age, arterial hypertension, arterial pressure on admission, atrial fibrillation, IHD, diabetes, smoking), C-reactive protein, free thyroxine, TSH, free T3 / free T4 ratio. Variables were introduced the regression model using the forward method.
In multivariate regression analysis showed that the independent factors affecting the severity of neurological deficiency in patients with no prior stroke were the level of free triiodothyronine (p = 0.0035) and the level of C-reactive protein (p = 0.0035). Model had the coefficient of variation F=17.45 (p < 0.0001) and corrected R2 = 0.49. The obtained results confirmed the independent effect of serum free triiodothyronine level on the severity of neurological deficit in newly developed ischemic stroke, while the low levels of triiodothyronine were associated with more severe stroke.
To assess the impact of the fT3 level on stroke outcome the multiple logistic regression method was used. Basic stroke risk factors (age, hypertension, blood pressure at admission, atrial fibrillation, coronary artery disease, diabetes mellitus, smoking) and C-reactive protein, free thyroxine, TSH levels, fT3 / fT4 ratio were included in the regression model. Lower fT3 levels were independently associated with poor stroke outcome (odds ratio = 0.3408, 95% CI 0.15 - 0.77), but this association became insignificant after correction for baseline SSS-score.
In order to determine the critical values of free triiodothyronine, associated with the greatest risk of poor stroke outcome, the analysis of ROC-curves was applied. It was established that level of fT3 < 4.44 pmol/l is a predictor of poor stroke outcome (AUC = 0.727, specificity - 96.4%, sensitivity - 66.8%, p = 0.003). The results of the analysis of ROC curves are presented in Figure 2.
Discussion
The results of this study indicate that low serum free triiodothyronine level during stroke onset is associated with a more severe neurological deficit in patient with first time ischemic stroke. The difference between this and analogical studies is the identification of independent influence of free triiodothyronine on the stroke severity in multivariate regression analysis. There are several limitations in this study. The main disadvantages are lack of long-term follow-up and inability to use Cox-regression method. This study was underpowered to reveal the independent influence of low free triiodothyronine level on poor stroke outcome. This may be attributed to relatively low sample size, short follow-up period and because the majority of patients were euthyroid. Nevertheless, obtained data showed trend for poorer outcome in patients with low free triiodothyronine levels (association of outcome and fT3 became insignificant only after correction for baseline SSS-score, but no other stroke risk factors). Our results correlate with the fundamental research carried out in recent years. In experimental models, it has been shown that the administration of L-thyroxin after transient cerebral ischemia contributes to an increase in neuron density and stimulation of angiogenesis in the ischemic brain [11]. The results of another in-vitro and research showed that triiodothyronine can recover intracellular concentration of natrium, calcium ions and pH [8]. It was shown that thyroxine stimulates the synthesis of other neurotrophic factors, such as fibroblast growth factor [12]. Several clinical studies also confirm experimental data. Lena M. O’Keefe et al. in a study of 868 patients with heterogeneous ischemic stroke found that a low triiodothyronine level was associated with a more functional deficiency in 3 months after a stroke and with more nosocomial mortality [13]. Results of another study of 833 patients with acute ischemic stroke, indicate that low levels of total triiodothyronine (even within the reference range) were associated with poor stroke outcome [14].
The biological basis of the association of hypothyroidism with the stroke course and outcome has not been finally studied. In this context, researches of new class of thyroid hormones – thyronamines (decarboxylated triiodothyronine derivatives) seems promising. These substances are a new class of endogenous signaling hormones that exhibit significant in-vivo effects, such as:
1. decrease in the rate of basal metabolism, decrease in respiratory rate [15];
2. fast, deep and reversible hypothermia [16];
3. weight loss [17];
4. negative inotropic and chronotropic effects without changing the absorption of glucose and oxygen [18];
5. decrease in Ca2+ level in the endoplasmic reticulum [19]
6. ischemia resistance [20];
7. increase in high-energy compounds level in target cells [21].
Thyronamines have a wide range of potential neuroprotective effects, the most significant of which are induction of hypothermia and a decrease in the rate of basal metabolism. According to Kristian P. Doyle et al., intraperitoneal administration of thyronamines to laboratory mice contributes to a decrease in body temperature from 37 to 31 Celsius degrees, and significantly reduces the infarction zone after transient cerebral ischemia. At the same time, the observed hypothermia is completely reversible and is not accompanied by any side effects, and its development is not associated with the effects of the known mechanisms of thermoregulation [22].
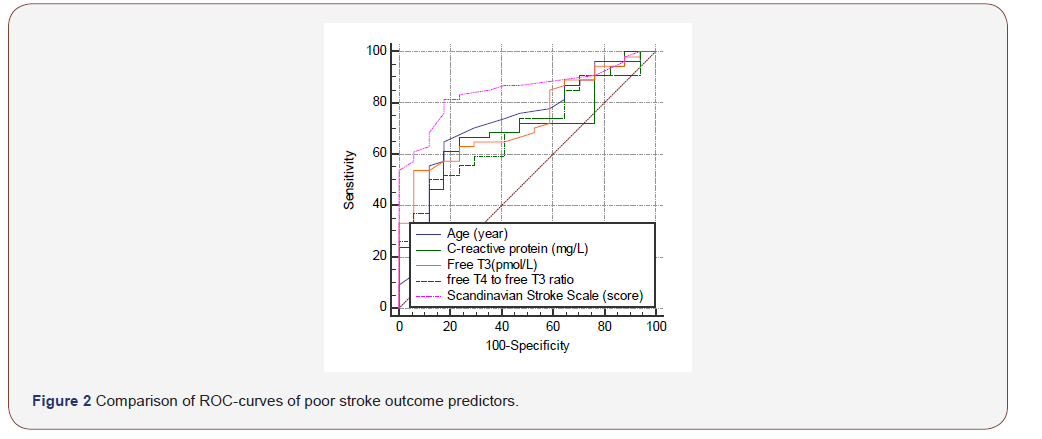
It is noteworthy that thyronamines, being derivatives of L-thyroxin, have antagonistic properties in relation to it, and the metabolic effects of thyronamines are close to the effects observed in hypothyroidism. It is possible that this phenomenon is the reason for the neuroprotective effects of thyroid hormones. To confirm the neuroprotective effects of thyronamines, analog of T0AM (thyronamine) was synthesized by our colleagues from the L.M. Litvinenko Institute of Physical Organic Chemistry and Coal Chemistry (Donetsk, Donbass region) for research in animal experimental model. Preliminary results showed that injection of T0AM in a dose of 50 mg / kg to laboratory rats (n=6) leads to a statistically significant decrease in the body temperature of experimental animals compared to controls (n=6), independently of other hypothermia agent (halothane). Body temperature dynamics after thyronamine injection is shown in Figure 3. Currently, the study of the thyronamine neuroprotective properties in the experimental stroke model is ongoing in our Institute (Figure 3).
Even though in recent years, more and more evidence has been obtained of the influence of thyroid hormones on risk, course and outcome of cerebrovascular disease, the nature and biological basis of this relationship have not been finally identified. This was the basis for initiation in our institute a complex clinical and experimental trial devoted to the study of the possibility of using thyroid hormones and their derivatives as neuroprotective therapy in acute ischemic stroke.
Conclusion
The study showed that a low serum free triiodothyronine level during stroke onset negatively affects the stroke severity in first-time stroke patients and may be predictor of its unfavorable outcome. There was a trend for association between low free triiodothyronine and unfavorable stroke outcome after 6 months. The findings suggest that thyroid metabolism is not only a factor affecting the course of ischemic stroke, but also a potential target for therapeutic correction. To confirm the effectiveness of this approach, it is necessary to conduct further clinical and experimental studies. It is reasonable to monitor thyroid hormone levels during stroke, while the analysis of serum free triiodothyronine can be used to predict a high risk of an unfavorable stroke outcome. Further researched are needed to determine possible beneficial effects of additional T3 supplements in ischemic stroke patients.
Acknowledgement
Our research team is sincerely grateful to the main scientific consultant Professor Stanislav Yevtushenko, director of the VK Gusak Institute of Urgent and Reparative Surgery Professor Emil Fistal, Deputy Director for Research of VK Gusak Institute of Urgent and Reparative Surgery Professor Oleg Antonyuk for critical comments and support. Special thanks to Tatyana Sautkina, head of the immunological laboratory of VK Gusak Institute of Urgent and Reparative Surgery.
Conflict of Interest
The authors declare that they have no competing interests.
References
- Stroke Association (2015) State of the Nation: Stroke Statistics 2015. State of the Nation: 39.
- IST-3 collaborative group, Sandercock P, Wardlaw JM, Lindley RI, Dennis M, et al. (2012) The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 379(9834): 2352-2363.
- Bhaskar S, Stanwell P, Cordato D, Attia J, Levi C (2018) Reperfusion therapy in acute ischemic stroke: Dawn of a new era? BMC Neurology 18(1): 8.
- Boyle K, Joundi RA, Aviv RI (2017) An historical and contemporary review of endovascular therapy for acute ischemic stroke. Neurovascular Imaging 3(1): 1.
- Liu R, Yuan H, Yuan F, Yang SH (2012) Neuroprotection targeting ischemic penumbra and beyond for the treatment of ischemic stroke [Internet]. Vol. 34, Neurological Research. Taylor & Francis: 331-337.
- Ramos-Cabrer P, Campos F, Sobrino T, Castillo J (2011) Targeting the ischemic penumbra. Stroke 2442-2456.
- Boltze J, Kleinschnitz C, Reymann KG, Reiser G, Wagner D-C, et al. (2012) Neurovascular pathophysiology in cerebral ischemia, dementia and the ageing brain - current trends in basic, translational and clinical research. Exp Transl Stroke Med 4(1): 14.
- Lin H-Y, Davis FB, Luidens MK, Mousa SA, Cao JH, et al. (2011b) Molecular Basis for Certain Neuroprotective Effects of Thyroid Hormone. Front Mol Neurosci 4: 29.
- Bunevicius A, Iervasi G, Bunevicius R (2015) Neuroprotective actions of thyroid hormones and low-T3 syndrome as a biomarker in acute cerebrovascular disorders. Expert Rev Neurother 15(3): 315-326.
- Forti P, Maioli F, Coveri M, Nativio V, Arnone G, Loreti A, et al. (2015) Thyroid function tests and early outcomes of acute ischemic stroke in older euthyroid patients. Exp Gerontol 61: 8-14.
- Schlenker EH, Schultz HD (2012) Hypothyroidism stimulates D2 receptor-mediated breathing in response to acute hypoxia and alters D2 receptors levels in carotid bodies and brain. Respir Physiol Neurobiol 180(1): 69-78.
- Sadana P, Coughlin L, Burke J, Woods R, Mdzinarishvili A (2015) Anti-edema action of thyroid hormone in MCAO model of ischemic brain stroke: Possible association with AQP4 modulation. J Neurol Sci 354(1–2): 37-45.
- O’Keefe LM, Conway SE, Czap A, Malchoff CD, Benashski S, et al. (2015) Thyroid hormones and functional outcomes after ischemic stroke. Thyroid Res 8(1): 9.
- Xu XY, Li WY, Hu XY (2016) Alteration of Thyroid-Related Hormones within Normal Ranges and Early Functional Outcomes in Patients with Acute Ischemic Stroke. Int J Endocrinol 2016: 3470490.
- Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, et al. (2004) 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med 10(6): 638-642.
- Venditti P, Napolitano G, Di Stefano L, Chiellini G, Zucchi R, et al. (2011) Effects of the thyroid hormone derivatives 3-iodothyronamine and thyronamine on rat liver oxidative capacity. Mol Cell Endocrinol 341(1–2): 55-62.
- Piehl S, Hoefig CS, Scanlan TS, Köhrle J (2011) Thyronamines - Past, present, and future. Endocr Rev 32(1): 64-80.
- Cichero E, Tonelli M (2017) New insights into the structure of the trace amine-associated receptor 2: Homology modelling studies exploring the binding mode of 3-iodothyronamine. Chem Biol Drug Des 89(5): 790-796.
- Chiellini G, Frascarelli S, Ghelardoni S, Carnicelli V, Tobias SC, et al. (2007) Cardiac effects of 3-iodothyronamine: A new aminergic system modulating cardiac function. FASEB J 21(7): 1597-1608.
- Kinne A, Kleinau G, Hoefig CS, Grüters A, Köhrle J, Krause G, et al. (2010) Essential molecular determinants for thyroid hormone transport and first structural implications for monocarboxylate transporter 8. J Biol Chem 285(36): 28054-28063.
- Panas HN, Lynch LJ, Vallender EJ, Xie Z, Chen GL, et al. (2010) Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice. J Neurosci Res 88(9): 1962-1969.
- Doyle KP, Suchland KL, Ciesielski TMP, Lessov NS, Grandy DK, et al. (2007) Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke 38(9): 2569-2576.
-
Runa B, Fei J, Rawan A R. Discovering Disrupted Connectivities in Neuroimaging Studies: A Meta Analytic Approach. Arch Neurol & Neurosci. 7(3): 2020. ANN.MS.ID.000661.
-
Ischemic stroke, Outcome, Thyroid hormones, Triiodothyronine, Acute Ischemic Stroke, Free Triiodothyronine, Free Thyroxine, Thyroid-Stimulating Hormone, C-Reactive Protein, Confidential Interval, Adenosine Diphosphate, Scandinavian Stroke Scale, Modified Rankin Scale.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






