 Research Article
Research Article
Intracerebral Hemorrhage (ICH) Score and the Prognosis of Spontaneous Intracerebral Hematoma
Damelan Kombate1, Komi Igneza Agbotsou2, Deborah Kaglan2, Kokou Mensah Guinhouya2, Agba Lehleng1*, Kossivi Apétsè2, Vinyo Kumako1*, Katanga Anthony Bekety1, Assogba Komi2, Mofou Bélo2 and Balogou AA Koffi2
1University of Kara, Regional Hospital of Kara, 1*Teaching Hospital of Kara, Togo
2Department of Neurology, University of Lomé, Teaching Hospital Campus, Togo
Damelan Kombate, University of Kara, Faculty of Health Science, Pobox : 404. Kara, Togo.
Received Date: August 13, 2021; Published Date: August 31, 2021
Abstract
Background: Intracerebral hemorrhage (ICH) score is a major predictor of 30-day lethality.
Purpose: The aim of this study was to investigate the lethality factors associated with cerebral hemorrhage, the correlations between lethality and the volume of the hematoma, the Glasgow score and the ICH score.
Methods: We conducted a retrospective and descriptive cross-sectional study of 604 patients admitted for an intracerebral hemorrhage (ICH) between January 1, 2014 and December 31, 2018 in the department of neurology of the Campus Teaching Hospital in Lomé. Patients with spontaneous ICH were included in this study. 68 files were excluded from the study because of lack of precision on clinical information and consequently 536 patients remained for the analysis. The ICH score was calculated according to the Hemphil formula. All statistical analyses were conducted using the Epi Data ® software version 4.6.0.2.
Results: The sex ratio M/F was 1.13 with a mean age of 51.9 years ± 12. Arterial hypertension was present in 70.19% and chronic alcoholism in (40.74%). The 30-day lethality was 25.93% (139/536). Increased ICH score was significantly correlated with greater lethality, p = 0.001. Hematoma volume was ≥ 30 ml in 5.78% (31/536) and <30 ml in 94.21% (505/536). Hematoma volume ≥ 30 ml, was significantly correlated with a greater lethality 54.54% (18/33) than a volume <30 ml, 23.88% (128/536), p = 0.001. The topography of the hematoma was supratentorial in 95.89% (514/536), subtentorial in 1.30% (7/536), basal ganglia 90.67% (486/536) and mixed in 2.05% (11/536).
Conclusion: Lethality associated with ICH remains high in our health centers. Our study has stressed the need to prevent ICH by screening and treating high blood pressure.
Keywords:Intracerebral haemorrhage; ICH score; Lethality, Togo
Introduction
Spontaneous intracerebral hemorrhage (ICH) or hemorrhagic stroke is an extravasation of blood in the brain parenchyma after a sudden and non-traumatic rupture of an intracranial vessel. ICHs are ranked second-leading cause of death in the world and in developing countries after cardiovascular diseases [1]. ICHs constitute 10 to 20% of strokes [2]. The risk of death at 30 days after intracerebral hemorrhage is assessed by calculating the ICH score established by Hemphill [3]. The evaluation of the ICH severity scores demonstrates a good correlation with the prognosis in its extreme values (≤ 1 and ≥ 4). On the other hand, no prognostic value was found with scores of 2 and 3. Other prognostic parameters such as Hunt and Hess score, Fisher scale, Modified Fisher scale, Glasgow score, Liege score, IGS2 score, hematoma volume, leukocytosis, hypernatremia on day 3, the increase in urea on day 3 above 65% of its initial value are also used to assess the severity of the cerebral hemorrhage [4]. In Togo, strokes are ranked first neurological disease. They represent more than 49% of hospitalizations [5]. The main purpose of this study was to analyze the lethality on the Hemphill ICH score in patients admitted for ICH between January 1, 2014 and December 31, 2018 in the department of neurology of the Campus Teaching Hospital of Lomé. The secondary objectives were to assess 30-day mortality, identify predictors of mortality and assess the life prognosis.
Subjects and Methods
A retrospective and descriptive single-center cross-sectional study of consecutive patients with ICH was undertaken.
Inclusion criteria
We included patients over 18 years of age on the date of January 1, 2014 who suffered from ICH through the topography technique proving the rupture of Charcot and Bouchard micro-aneurysms, confirmed by brain imagery and admitted to the department. A computed tomography (CT) was performed on D0 and repeated at different times after the onset of symptoms. During this period, 5,741 patients with stroke were admitted including 2129 with ICH. Of the 2129, 85 patients had been transferred to the department of neurosurgery with a surgical indication. Among patients with a medical management, 604 cases were analyzed.
Exclusion criteria
We excluded ICHs whose topography do not present hypertension especially ICHs related to tumors, cerebral venous thrombosis, cavernoma or trauma, subarachnoid, subdural and extra-dural hemorrhages and ICHs whose history was unknown. Of the 604 complete files with the diagnosis of cerebral hemorrhage, 68 were excluded. A total of 536 files were examined in this study.
Calculation of volume hematoma and ICH score
Hematoma volume was measured by the product of the largest diameter of the hematoma in centimeters on a sagittal, axial and coronal section divided by 2. The ICH score was calculated according to the Hemphill formula (3). Hematoma volume was measured according to the formula ABC/2 (A represented the length of the hematoma in centimeters, B, the width in centimeters and C, the thickness). We used the Glasgow score to assess the Consciousness. Consciousness was normal for a score of ≥13, coma stage I for a score of 8-12, stage II between 6-7, stage III between 4-5 and stage IV for a score of 3.
Statistical analysis
The collected data were entered into the EpiData ® software version 4.6.0.2. The data development and validation were carried out to detect entry errors and correct them. Data analysis was done using Stata ® software version 15. Quantitative variables were presented by their mean (standard deviation) or median (inter quartile interval). Qualitative variables were described by their absolute and relative frequency. The chi-squared test and Fisher’s exact test were used for the comparison of percentages. The significant threshold was 0.05.
Results
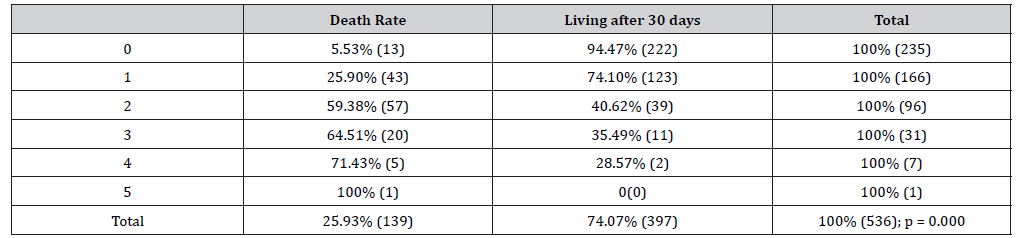
From the patients enrolled in the study, male to female ratio represented 1.13. Patient mean age was 51.9 ± 12.13 years (range, 17 to 94 years) with 30.56% (range, 50 to 60 years). Subjects over 55 years were 40.19%. The admission mean time was 12 hours with an interquartile range of [4h; 48h]. The major risk factors were dominated by hypertension (70.19%), chronic alcoholism (40.74%), diabetes (8.33%), tobacco (5.00%), antiplatelet therapy (4, 63%), a personal history of stroke (4.63%), a family history of stroke (4.63%), sedentarity (1.48%), drepanocytosis (1.48%). According to the stage of the coma (Table 1), consciousness was normal in 58.39% (313/536). At the admission, 91.04% (488/536) had a blood pressure ≥ 140 mm Hg systolic. Neurological symptoms were dominated by: signs of intracranial hypertension (37.78%), headache (20.93%), convulsive seizures (9.26%), hemicorporeal motor deficit (84.26%), speech disorders (31.48%), meningeal signs (18.33%) and oculomotor palsy (5.74%). The hemorrhage topography was supratentorial 95.89% (514/536), sub tentorial 1.30% (7/536), mixed 2.05% (11/536) and at basal ganglia 90, 67% (486/536). The mean volume of hematoma was 2.1 ml and the extremes were 0.01 ml and 158 ml. Mass effect, brain engagement and ventricular flooding were present in 79.27%, 44.97% and 34.54% respectively. Biologically, 45.19% of patients presented hyperglycemia. The mean time of patient stay was 9 days and it ranges from 0 to 30 days. 25.93% (139/536) died during hospitalization. For an age ≥ 55 years, the death rate was 28.7% (62/536) and 23.99% (77/536) died at an age <55 years (p = 0.221). The mortality rate for men was 29.92% (85/284) and 21.43% (54/252) for women (p = 0.027). Increased ICH score (Table 2) was significantly correlated with greater lethality (p = 0.001). For systolic blood pressure ≥140 mm Hg, the lethality was 27. 66% (135/488) and the mortality was 10.41% (5/48) for blood pressure <140 mm Hg (p = 0.010). The hematoma volume was ≥ 30 ml in 5.79% (31/536), and <30 ml in 94.21% (505/536). Hematoma volume ≥30 ml (54.54% (18/33)) was significantly correlated with higher mortality than a volume <30 ml, 23.88% (128/536) (p = 0.001). With an ICH score of 1, there was a mortality of 25.90% and with an ICH score of 4, there was a lethality of 71.43% (p = 0, 000). The ICH was associated with hyperglycemia 45.19% (244/536), biological inflammatory syndrome 33.39% (179/536), elevated transaminases 26.30% (142/536), polycythemia 7.96% (43/536), anticoagulant treatment 2.78% (15/536) and HA1Ac at 6% in 1.85% (10/536).
Table 1:Lethality and Glasgow Coma Stage.
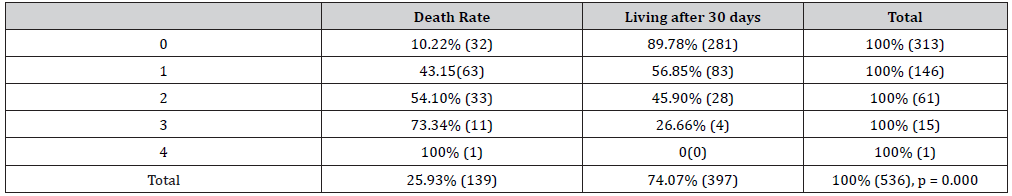
Table 2:ICH Score andLethality.
Discussion
This study was not free from some limitations. First, the retrospective method did not allow to establish the correlation in the evolution of Glasgow score, blood pressure, hematoma volume with mortality. Second, the presence of small centers which take care of strokes affect the 30-day mortality rates for patients with ICH observed in the center of this study that represents the largest center for stroke care in the country. However, the study established a clear relationship between the ICH score, the volume of the hematoma, the Glasgow score and the mortality associated with ICH. The increase in health needs in developing countries is not followed by the increase in material resources, infrastructure and personnel. The major risk factors for ICH were hypertension (70.19%) and chronic alcoholism (40.74%). Although the mechanism of action remains controversial, daily consumption of alcohol increases vascular risk for men, consuming more than 4 drinks on any day or more than 14 drinks per week and for women, consuming more than 3 drinks on any day or more than 7 drinks per week [6,7]. Alcoholism was difficult to analyze in the study because of the impossibility to quantify the degree of alcohol consumed in our context where patients consume either traditional alcohol or a mixture of several types of alcohol. At a certain degree, alcoholism could cause an increase in blood pressure and an absence of alcohol consumption could reduce blood pressure levels. However, there is a genetic predisposition to alcoholism [8,9]. A combination of factors aggravates the occurrence of ICH. They involve undiagnosed and untreated hypertension, a delay in admission (mean time = 12 hours) to the hospital and a lack of technical support for treatment [10,11]. Several elements should be taken into account during the acute phase of ICH. The blood pressure must be reduced below 140 mm Hg. Blood pressure reduced to 140-135 mm Hg within 24 hours reduces the volume of hematoma and improves vital and functional prognosis [12,13]. Blood pressure was said for a long time to promote cerebral perfusion but today studies show that it is directly correlated with the volume of hematoma in the acute phase of ICH. However, if there is a consensus on the reduction in hematoma volume and the reduction in blood pressure in the acute phase, the influence of this reduction on the overall prognosis of ICH is questioned by some scholars [14]. The prognosis of cerebral hemorrhage can be improved by a set of measures especially blood sugar in acute phase between 80 - 110 mg / dl, temperature in particular hypothermia which is an element likely to improve the prognosis. Hyperthermia is a factor of poor prognosis [15]. In fact, by reducing the production of free radicals harmful to the cell, hypothermia reduces the oxygen consumption of the neuron by reducing metabolic phenomenon [16]. The C-reactive protein may be a major prognostic element in hematoma expansion [17,18]. The prognosis of ICH also depends on the unit of medical care. Patients in this study were taken care in a neurovascular emergency health center with very little technical support. A neurovascular emergency health center is a guarantee of a better prognosis than an intensive care health center that is not specialized in neurovascular [19]. The acute hyperglycemia was 45.19%. With regard to the risk factors, arterial hypertension is the first cause with particular topographies which prove the rupture of the microaneurysms of Charcot and Bouchard especially in the central gray nuclei (90.67%) in the cerebellum and on the internal capsule. The specification of the duration of the development of arterial hypertension in our patients was difficult because the majority of subjects are diagnosed during a first stroke [20]. In 9.26%, there were epileptic seizures at the start of symptoms. An epileptic seizure can occur during the onset of the ICH. However, it does not determine vascular epilepsy which is rather determined after 14 days of progression [21]. While certain topographies proved surgery in the ICH, patients whose CT appearance met the criteria for surgery were transferred upon admission to neurosurgery. In acute hydrocephalus, surgery is indicated either by intraventricular hemorrhage or by compression of the cerebrospinal fluid outflow pathways or craniectomy to decompress a hemispherical hematoma [22]. Hematomas of the cerebellum with compression of the Sylvian aqueduct or the fourth ventricle or compressive lobar hematomas are indicated for surgery. Surgery for hematoma was not effective [23].
Conclusion
Mortality from cerebral hemorrhage is high. The ICH score and the Glasgow scale are major prognostic elements. Intracranial hypertension is the likely leading direct cause of death. However, several mortality factors could be associated in the same patient due to the comorbidities. The main risk factors are high blood pressure and chronic alcoholism. Care remains essentially medical and could be diversified into surgical care for hemorrhages with specific criteria. Hypertension treatment and the reduction of alcohol consumption are important to reduce mortality due to ICH. The management of ICH remain very difficult in case of poor technical platform.
Acknowledgments
Not applicable
Conflict of interest
No conflict
References
- Lanas F, Seron P (2021) Facing the stroke burden worldwide. Lancet 9(3): E235-E236.
- Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, et al. (2019) Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: 357–375.
- Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC (2001) The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 32: 891-897.
- Kariman H, Hatamabadi H, Shojaee M, Asarzadegan F, Saljughi S (2019) Validation of SUSPEKT Score in Predicting One-month Mortality of Patients with Hemorrhagic Stroke; a Diagnostic Accuracy Study. Arch Acad Emerg Med 7(1): e56.
- Yusuf S, Joseph P, Rangarajan S, Shofiqul Islam, Andrew Mente, et al. (2020) Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 395: 795-808.
- Roerecke M, Kaczorowski J, Tobe SW, Gmel G, Hasan OSM (2017) The effect of a reduction in alcohol consumption on blood pressure: a systematic review and meta-analysis. Lancet Public Health 2: e108-e120.
- Jialing Peng, Hongxuan Wang, Xiaoming Rong, Lei He, L Xiangpen (2020) Cerebral Hemorrhage and Alcohol Exposure: A Review. Alcohol and Alcoholism 55(1): 20–27.
- Millwood IY, Walters RG, Mei XW, Guo Y, Yang L, et al. (2019) China Kadoorie Biobank Collaborative Group. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet 393: 1831–1842.
- Susanna C Larsson, Stephen Burgess, Amy M Mason, Karl Michaëlsson (2020) Alcohol Consumption and Cardiovascular Disease. A Mendelian Randomization Study. 2020Circulation: Genomic and Precision Medicine 13: e002814.
- Mayowa O Owolabi, Amanda G Thrift, Sheila Martins, Walter Johnson, Jeyaraj Pandian, et al. (2021) The state of stroke services across the globe: Report of World Stroke Organization-World Health Organization surveys. Int J Stroke: 17474930211019568.
- Avan A, Digaleh H, Di Napoli M, Saverio Stranges, Reza Behrouz, et al. (2019) Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study BMC: 191.
- Lin J, Piran P, Lerario MP, Ong H, Gupta A, et al. (2020) Differences in admission blood pressure among causes of intracerebral hemorrhage. Stroke 51(2): 644-647.
- Biffi A, Anderson CD, Battey TW, Ayres AM, Greenberg SM, et al. (2015) Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA 314(9): 904-912.
- Cordonnier C, Demchuk A, Ziai W, Anderson CS (2018) Intracerebral haemorrhage: Current approaches to acute management. Lancet 392: 1257–1268.
- Shoamanesh A, Patrice Lindsay M, Castellucci LA, Cayley A, Crowther M, et al. (2021) Canadian stroke best practice recommendations: Management of Spontaneous Intracerebral Hemorrhage, 7th Edition Update 2020. Int J Stroke 16(3): 321-341.
- Loggini A, Ammar FE, Awad IA, Lazaridis C, Kramer CL, et al. (2020) Temporal Evolution and Outcomes of Non-Traumatic Intracerebral Hemorrhage in Hospitalized Patients. J Stroke Cerebrovasc Dis 30(3): 105584.
- Bernstein JE, Savla P, Dong F, Zampella B, Wiginton JGT, et al. (2018) Inflammatory markers and severity of intracerebral hemorrhage. Cureus 10: e3529.
- Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW (2014) Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol 115: 25–44.
- Peter Langhorne, Patricia Fearon, Ole M Ronning, Markku Kaste, Heikki Palomaki, et al. (2013) Stroke Unit Care Benefits Patients with Intracerebral Hemorrhage Systematic Review and Meta-analysis. Stroke 44: 3044–3049.
- Anayo KN, Agba L, Guihouya KM, Codjia V, Kombate D, et al. (2017) Facteurs prédictifs de mortalité des hématomes cérébraux aux CHU de Lomé. African Journal of Neurological Sciences 36: 17-22.
- Räty S, Sallinen H, Virtanen P, Haapaniemi E, Wu TY, et al. (2021) Occipital intracerebral hemorrhage-clinical characteristics, outcome, and post-ICH epilepsy. Acta Neurol Scand 143(1): 71-77.
- Polster SP, Carrión-Penagos J, Lyne SB, Gregson BA, Cao Y, et al. (2021) Intracerebral Hemorrhage Volume Reduction and Timing of Intervention Versus Functional Benefit and Survival in the MISTIE III and STICH Trials. Neurosurgery 88(5): 961-970.
- Gross BA, Jankowitz BT, Friedlander RM (2019) Cerebral Intraparenchymal Hemorrhage: A Review. JAMA 321(13): 1295-1303.
-
Damelan Kombate, Komi Igneza Agbotsou, Deborah Kaglan, Kokou Mensah Guinhouya etc all.. Intracerebral Hemorrhage (ICH) Score and the Prognosis of Spontaneous Intracerebral Hematoma. Arch Neurol & Neurosci. 11(2): 2021. ANN.MS.ID.000756.
-
Intracerebral Haemorrhage, ICH Score, Lethality, Togo, High Blood Pressure, Chronic Alcoholism, Neurology, Brain Parenchyma, Cardiovascular Diseases, Hypernatremia, Leukocytosis.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






