 Research Article
Research Article
Inpatient Rehabilitation Admission Rate and Functional Improvement in Pediatric Patients with Autoimmune Encephalitis
Jussely Morfin1*, Katherine Thomas2, Arshad Ali1, Benjamin Dirlikov1, Yumi Mitsuya3 and Thao Duong1,4
1Rehabilitation Research Center, Santa Clara Valley Medical Center, San Jose, CA, USA
2Department of Pediatric Rehabilitation Medicine, Gillette Children’s Specialty Healthcare, St Paul, MN, USA
3Pediatric Rehabilitation Medicine Section, Department of Orthopedic Surgery, UCSF Benioff Children’s Hospital Oakland, CA, USA
4Department of Physical Medicine and Rehabilitation, Santa Clara Valley Medical Center, San Jose, CA, USA
Morfin, Rehabilitation Research Center (RRC), Santa Clara Valley Medical Center, 751 S. Bascom Ave, San Jose, CA 95128, USA.
Received Date: July 28, 2022; Published Date: September 14, 2022
Abstracts
This study aimed to investigate the inpatient rehabilitation admission rate and the degree of functional improvement made during the course of acute inpatient rehabilitation (AIR) among pediatric patients with autoimmune encephalitis (AE). A retrospective chart review was conducted for pediatric patients admitted to the study site (01/2014-03/2018) with a diagnosis of AE. Data obtained from chart review focused on the transition of patients diagnosed with AE on the general pediatric service to AIR as well as functional improvements as measured with Functional Independence Measure for Children (WeeFIM) from AIR admission to discharge. The prevalence of AE within the general pediatrics admissions was 0.2%, of which 94% (16/17) were referred and admitted to AIR. Patients with AE who underwent AIR (length of stay: median[range] = 8 [5-149] days) showed significant improvements in thirteen out of the eighteen WeeFIM items (p<.027). Nine WeeFIM items showed functional improvements representing transitions from complete dependence on AIR admission to modified independence at discharge. Although results from this cohort point to the benefits of AIR for pediatric patients with autoimmune encephalitis, larger studies are needed to understand factors associated with improvements observed during AIR.
Keywords:Autoimmune encephalitis; Functional outcomes; Functional independence measure; Pediatric rehabilitation; Children and adolescents
Keywords:AIR: Acute Inpatient Rehabilitation; AE: Autoimmune Encephalitis; NMDA Receptor: N-Methyl D-Aspartate Receptor; ADEM: Acute Disseminated Encephalomyelitis; ADHD: Attention- Deficit/Hyperactive Disorder; IRB: Institutional Review Board; PM&R: Physical and Medical Rehabilitation Department; IVIG: Intravenous Immunoglobulin; LOS: Length of Stay; PT: Physical Therapists; OT: Occupational Therapists; SLP: Speech-Language Pathologists
Introduction
Encephalitis is a disease caused by acute inflammation of the brain tissue [1]. Although infectious etiologies (e.g., viral, bacterial, fungal, parasitic) of encephalitis have long been recognized, more recent discoveries and improved diagnostic testing have made autoimmune encephalitis (AE) gain recognition [2]. The estimated prevalence for children and adults with encephalitis and AE is 11.6 per 100,000 persons and 13.7 per 100,000 persons, respectively [3]. Incidence of encephalitis from infectious etiologies is comparable in pediatric and adult populations (10.5 per 100,000), whereas AE is observed less in children than in adults (1.54 per 1,000,000 children compared to 1.2 per 100,000 adults) [4,5].
AE is an acquired disorder in which the immune system, including autoreactive lymphocytes or auto-antibodies, targets the CNS, causing neurologic dysfunction [6]. Subtypes of AE include NMDA receptor (N-methyl D-aspartate receptor) and ADEM (Acute disseminated encephalomyelitis), in which pathogenic autoantibodies bind to neuronal cell surface proteins such as receptors, ion channels, or synaptic proteins [7]. AE is often preceded by a mild viral-like illness, though some paraneoplastic syndromes have also been described, as in the case of NMDA receptor encephalitis [8].
Clinically, AE can present with headache, fever, cognitive deficits, behavioral changes, seizures, dyskinesias, autonomic dysfunction, and decreased consciousness [9]. Given the nonspecific nature of its symptoms, AE can initially be difficult to differentiate from other conditions such as psychiatric disorders and central nervous system infections [10]. Furthermore, young children may be limited in their ability to communicate their symptoms or may be unable to cooperate with examination, which can contribute to difficulty in diagnosing pediatric AE [10]. Current available immunological tests for autoimmune encephalitis includes 25 autoantibodies for serum samples and 21 autoantibodies for CSF samples [11]. It is important to note, however, that a large proportion of patients with suspected AE are seronegative [12]. The current acute treatment approach to proven or suspected AE includes removal of possible underlying triggers, such as malignancy or infection, and early initiation of immune modulating therapy, which can include high dose IV corticosteroids, intravenous immunoglobulin (IVIG), and/ or plasma exchange [13–15].
Even after diagnosis and appropriate initiation of treatment, pediatric AE is still known to cause significant morbidity including moderate to severe functional deficits in many cases [16,17]. The impact of these functional deficits can be amplified in part due to AE’s often protracted disease course, which can result in prolonged hospitalization and recovery [18]. Disease burden also stems from incomplete recovery with many patients having residual functional deficits, such as reduced neurocognitive performance, attention deficits, impulsivity, and behavioral disorders [18,19]. The goal of early rehabilitation in AE patients is not only to improve mobility and functional status, but also to mitigate long term morbidity from these neurocognitive and behavioral problems. Studies have indicated that children with AE demonstrate functional gains with rehabilitation, particularly in areas of self-care, cognition, and mobility, although many patients still demonstrate prolonged residual deficits [20,21]. As such, there remains a need to better understand the range of functional deficits that AE causes in children, the recovery process after presentation and treatment, and how different rehabilitation strategies may improve functional outcomes.
This retrospective study aimed to investigate the AIR referral rate and degree of functional improvement among pediatric patients who received AIR for AE. Specifically, the hypotheses of the study were that 1) the majority of patients admitted to the general pediatrics service with a diagnosis of AE had persistent neurological and functional deficits after medical treatment that warranted AIR referral and 2) AE patients would demonstrate significant functional gains during AIR as quantified by improvement in WeeFIM scores - a validated functional outcome assessment tool for pediatric patients [22].
Materials and Methods
Chart review
This retrospective chart review was approved by the local Institutional Review Board (IRB). The study reviewed patient charts from January 2014 – March 2018, which encompassed the start of the electronic medical record at the study site to the IRB submission date. Patients who met inclusion criteria were between the ages of 2- 18 years old and required a referral to Physical and Medical Rehabilitation Department (PM&R) for AIR with a diagnosis of AE following their acute inpatient hospitalization with the general pediatrics service. Only patients who received AIR for a new diagnosis of AE were included in this review; re- referrals were not included in the study. Data was extracted from the local electronic medical record system (Epic®). Two independent researchers reviewed relevant medical records for data extraction. A set of screening criteria for possible AE diagnosis was created to review charts of individuals admitted to the general pediatric service (Table 1).
Table 1:Screening Criteria for Possible AE Diagnosis.
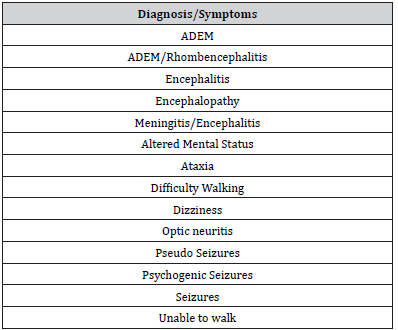
Confirmation of AE diagnosis required at least 3 minor criteria which included a fever within 72 hours of presentation, new onset focal neurologic findings, CSF leukocytosis, acute new neuroimaging abnormality suggestive of encephalitis, or EEG abnormalities consistent with encephalitis. Medical records were manually screened for a possible AE diagnosis if any of the following discharge diagnoses, symptoms, or treatments were noted: acute disseminated encephalomyelitis (ADEM), rhombencephalitis, altered mental status, ataxia, difficulty walking, encephalitis, encephalopathy, optic neuritis, epileptic seizures, non-epileptic seizures, inability to walk, weakness, rehabilitation, and IVIG. Two reviewers independently abstracted data and then compared the results to ensure reliability. Any disagreements between the reviewers were resolved through discussion until a consensus was reached.
Extracted data
Clinical features: demographics and clinical data: For all patients with a confirmed AE diagnosis, clinical data abstracted included: diagnosis, age, assigned sex at birth, race/ethnicity, length of stay (LOS) on the general pediatrics service and on the AIR service, hospital notes, type of immunomodulatory therapy received for encephalitis (e.g., IVIG, steroids, plasmapheresis), type of antibody associated with encephalitis (if identified), neuroimaging findings, and disposition.
Functional outcomes: WeeFIM: The Functional Independence Measure for Children (WeeFIM) scores were abstracted for all patients included in this study [22]. The WeeFIM instrument is a validated tool developed for use in children 6-months to 7-years of age with application through adolescence. The WeeFIM has been widely used to quantify functional performance and can be used to track pediatric functional improvements [23]. Specifically, functional outcomes tracked by WeeFIM can be divided into three domains: 1) self-care, 2) mobility, and 3) cognition. Within a domain, each individual item is scored based on a scale ranging from 1-7; a score of 6-7 (No Helper) reflects that an individual is independent, a score of 3-5 (Helper: Modified Independence) indicates the need for some caregiver assistance, and a score of 1- 2 (Helper: Complete Dependence) signifies complete dependence on a caregiver for a particular functional task. A score of 0 signifies no score or data was not available to assign a score [22]. When available, WeeFIM scores within the electronic medical record were directly abstracted used in this study. If a patient’s WeeFIM scores were not recorded in the medical record, the study team calculated the remaining WeeFIM scores using the WeeFIM guidelines and medical chart review. Information from the medical chart review included notes from physicians, physical therapists (PT), occupational therapists (OT) and speech-language pathologists (SLP), within a week of acute rehabilitation admission and discharge. The bowel and bladder accident scores were based on any accidents within 7 days of admission or discharge. If patients required intermittent catheterization for urination or were more than 5 years old, they were scored as total assist (i.e., WeeFIM score of 1). This age was chosen as previous literature has confirmed that some children as young as 5 years old can be taught to self-catheterize, as clinically appropriate. [24] Locomotion scores were based on a child’s primary mode of locomotion. For all other WeeFIM items, the lowest score available within 3 days of admission and 3 days of discharge was used. If there was insufficient information, the WeeFIM item would be scored as 0. Due to the retrospective nature of this study and insufficient information to assign scores, 20% of items were scored 0. To ensure WeeFIM score reliability a rehabilitation physician and a research assistant, both certified in scoring WeeFIM, independently scored each patient’s WeeFIM. WeeFIM score discrepancies were first reviewed, and a consensus was reached. A third WeeFIM certified assessor scored any patient items for which the initial two assessors could not reach consensus.
Data analysis
Summary statistics for demographic characteristics (e.g., age, assigned sex at birth, length of stay) for all AE patients during the study period were calculated. Additionally, the number of patients referred to PM&R and their WeeFIM scores were summarized as counts and percentages. Mann-Whitney U tests were used to compare LOS of individuals with AE to all other patients admitted to general pediatrics and AIR services separately. To assess the functional changes from admission to discharge for individuals with AE, uncorrected Wilcoxon sign rank tests for each WeeFIM category were used. Due to incomplete WeeFIM data for several participants, total WeeFIM scores were not included in the analyses and the sign rank tests only included scored items (i.e., items with non-zero scores).
Results and Discussion
Demographics and clinical characteristics
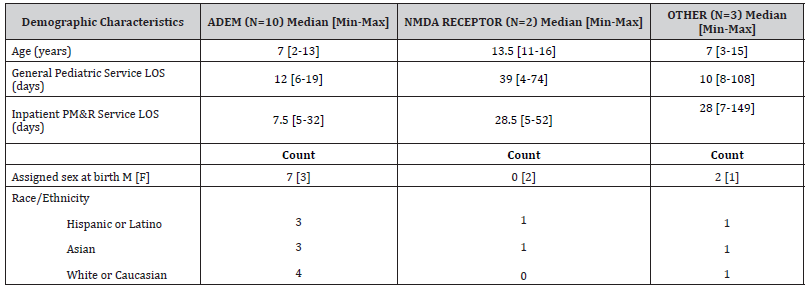
From January 2014 to March 2018, there were 8,206 patients admitted to the general pediatrics service, of which 17 met criteria for AE. One patient was not referred to PM&R due to no significant neurologic deficits, while the remaining 16/17 (94%) patients were referred and admitted by the PM&R Department for AIR. Furthermore, one additional patient was excluded from analysis due to not completing the full course of AIR. The median age of all pediatric patients admitted to the AIR service was 9 years old [Range=2-18 years], while the median age of AE patients receiving AIR was 8 years old [Range=2-16 years] (Mann-Whitney U = 807, p = .257). Table 2 shows median age, LOS on the pediatric service and AIR as well as race/ethnicity and assigned sex at birth demographics (Table 2).
Table 2:Demographic characteristics of AE patients undergoing AIR.
Functional outcomes
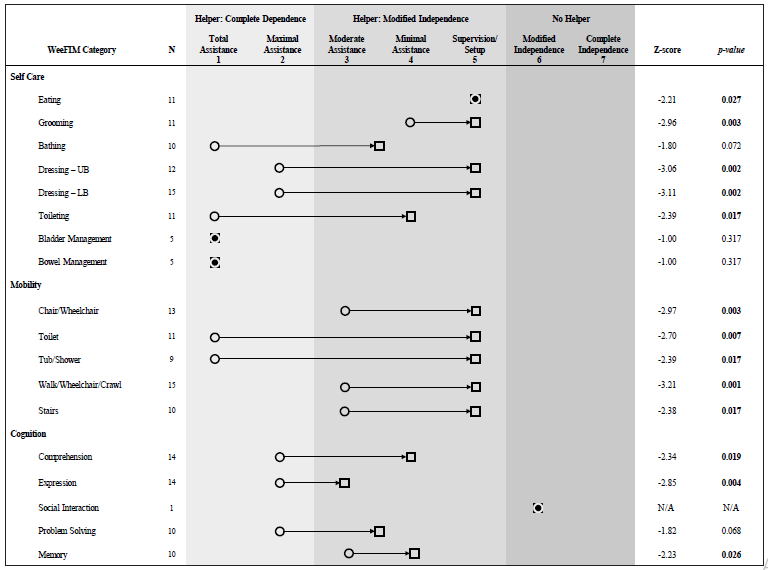
The WeeFIM itemized analysis revealed significant uncorrected improvements from admission to discharge in self-care, mobility, and cognition categories (Figure 1). Specifically, significant gains were observed in the following self -care items: eating (N=11, Z=- 2.21, p=0.027), grooming (N=11, Z=-2.96, p=0.003), upper body dressing (N=12, Z=-3.06, p=0.002), lower body dressing (N=15, Z=- 3.11, p=0.002), and toileting (N=11, Z=-2.39, p=0.017). Significant improvements in mobility items were observed in chair/wheelchair (N=13, Z=-2.97, p=0.003), toilet transfers (N=11, Z=-2.70, p=0.007), tub/shower transfers (N=9, Z=-2.39, p=0.017), walk/wheelchair/ crawl (N=15, Z=-3.21, p=0.001), and stairs (N=10, Z=-2.38, p=0.017). Significant gains were also observed in the cognition items for comprehension (N=14, Z=-2.34, p=0.019), expression (N=14, Z=- 2.85, p=0.004), and memory (N=10, Z=-2.23, p=0.026). The changes that occurred during AIR for the remaining WeeFIM items including bathing, bladder and bowel management, and problem solving did not reach statistical significance (p > 0.068). The social interaction item scores were not analyzed because only one patient had admission and discharge data; this participant showed no change in their social interaction score.
Discussion
In this investigation, it was observed that 0.2% (17/8206) of patients admitted to the general pediatrics service were diagnosed with AE, of whom approximately 94% (16/17) were referred for AIR. Although our chart review revealed a small number of patients with AE, these patients nonetheless provide insights into the functional recovery during AIR. While on the AIR service, patients in our sample achieved significant improvements in multiple areas of functioning as demonstrated by increased median WeeFIM score across 13 items. The information from this study may help clinicians better understand the expected disease course and provide better counsel for patients and families with AE.
Among pediatric patients hospitalized with AE, functional deficits and eventual functional treatment-related improvements are only recently becoming more characterized in the literature, although research in this area remains limited [21,25,26]. The largest NMDA receptor encephalitis study to date (N=27) by Howarth et al. (2019) has highlighted the heterogeneity in presenting symptoms and immunomodulatory treatment in pediatric patients with NMDA receptor encephalitis [21]. Despite this heterogeneity, Howarth et al. (2019) showed patients had significant functional gains during AIR in their cohort of 27 pediatric patients [21]. Additionally, a large retrospective study by Bagdure, et al. in 2016 that included 7298 children with encephalitis of any cause, reported that following hospitalization for encephalitis, only 3% of patients (N=228) were transferred to a rehabilitation center [27]. As only 17% of patients in the Bagdure et al. study were treated with IVIG and only 4% were treated with plasmapheresis, a large proportion of this cohort may have had an infectious cause of encephalitis, which upon treatment of the infection may have resulted in a return to baseline neurological function, negating the need for inpatient rehabilitation [27]. Bagdure’s findings are in contrast to this study’s cohort of patients with autoimmune encephalitis, where 94% (N=16) of AE patients were referred for AIR. The high referral rate in this study may have been due to the PM&R service having a strong presence at this institution and a high percentage of patients having a level of functional deficits that may benefit from AIR. Specifically, patients in this study on average were categorized as complete dependence on 11/18 WeeFIM items at AIR admission.
This study also aimed to provide information regarding functional improvements of pediatric patients with AE during AIR. The significant improvements in WeeFIM scores across all domains (self-care, mobility, and cognition) during AIR is encouraging, especially given that many patients were able to progress from being largely dependent for care to requiring modified independence (Figure 1).
Patients showed no significant progress in tasks such as bathing and bowel/bladder management, requiring total assistance at both admission and discharge. However, despite these significant functional improvements throughout their stay, most patients still required some type of assistance at the time of discharge, highlighting the importance of continued outpatient rehabilitation and need for further research on functional changes in AE patients after discharge.
This study was limited by several factors including the inherent weaknesses associated with a retrospective study design. Primarily missing data presented an issue where the total WeeFIM score could not be calculated. Additionally, although the WeeFIM has been widely used by other researchers to quantify functional levels, this scoring system minimizes the complexity of functional status across individual patients. Furthermore, WeeFIM scores can vary greatly even among neurodevelopmentally typical children of different ages, which can make comparing WeeFIM scores among children of varied ages difficult. This study had a sample size N=15 which also limited the study’s ability to investigate differences in AE subgroups (for example ADEM compared to NMDA receptor groups). This study was conducted at a single center, which also limits the generalizability of the results.
Despite the above limitations, the observed WeeFIM improvements seen in the current study can provide a starting place for additional research into pediatric functional outcomes after AE. In order to better understand the optimal rehabilitation regimen to maximize functioning after AE, prospective, randomized, controlled, multisite studies with larger sample sizes would be necessary. Moreover, there is a need for additional research that can better adjust for expected age-related changes in WeeFIM when comparing cohorts of children across the age spectrum. This improved evidence-based understanding of the interventions needed to maximize functional recovery can in turn assist families and clinicians in advocating for rehabilitation services needed by pediatric AE patients to reach their potential both in childhood and beyond.
Conclusion
This study aimed to investigate the AIR referral rate and degree of functional improvement among pediatric patients receiving AIR for AE. In this sample, AE affected 0.2% of patients admitted to the general pediatrics service, of which 94% were referred for AIR. On average, patients with AE who underwent AIR showed significant functional gains as demonstrated by improvement in thirteen out of the eighteen WeeFIM items. Despite these significant functional improvements, most patients still required some caregiver assistance at the time of discharge, highlighting the importance of continued rehabilitation in the outpatient setting. Given the significant disability associated with AE, further research into interventions to maximize functional outcomes is warranted.
Acknowledgements
There are no acknowledgements.
Conflict of Interest
There are no conflicts of interest.
References
- Mennella H, Smith N (2016) Encephalitis in Children [Internet]. Cinahl Information Systems.
- Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, et al. (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15(4): 391-404.
- Dubey D, Pittock SJ, Kelly CR, McKeon A, Lopez-Chiriboga AS, et al. (2018) Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis: Autoimmune Encephalitis. Ann Neurol 83(1): 166-77.
- Koskiniemi M, Korppi M, Mustonen K, Rantala H, Muttilainen M, et al. (1997) Epidemiology of encephalitis in children. A prospective multicentre study. Eur J Pediatr 156(7): 541-545.
- de Bruijn MAAM, Bruijstens AL, Bastiaansen AEM, van Sonderen A, Schreurs MWJ, et al. (2020) Pediatric autoimmune encephalitis: Recognition and diagnosis. Neurol Neuroimmunol Neuroinflamm 7(3): e682.
- Dale RC, Gorman MP, Lim M (2017) Autoimmune encephalitis in children: clinical phenomenology, therapeutics, and emerging challenges. Curr Opin Neurol 30(3): 334-344.
- Dalmau J, Geis C, Graus F (2017) Autoantibodies to Synaptic Receptors and Neuronal Cell Surface Proteins in Autoimmune Diseases of the Central Nervous System. Physiol Rev 97(2): 839–87.
- Miya K, Takahashi Y, Mori H (2014) Anti-NMDAR autoimmune encephalitis. Brain and Development. 36(8): 645–652.
- Shim Y, Kim SY, Kim H, Hwang H, Chae J-H, et al. (2020) Clinical outcomes of pediatric Anti-NMDA receptor encephalitis. Eur J Paediatr Neurol 29: 87-91.
- Cellucci T, Van Mater H, Graus F, Muscal E, Gallentine W, et al. (2020) Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol Neuroimmunol Neuroinflamm 7(2) : e663.
- Lee SK, Lee S-T (2016) The Laboratory Diagnosis of Autoimmune Encephalitis. J Epilepsy Res 6(2): 45-50.
- Hacohen Y, Wright S, Waters P, Agrawal S, Carr L, et al. (2013) Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry 84(7): 748-755.
- Vedeler CA, Antoine JC, Giometto B, Graus F, Grisold W, et al. (2006) Management of paraneoplastic neurological syndromes: report of an EFNS Task Force. Eur J Neurol 13(7): 682-690.
- Thieben MJ, Lennon VA, Boeve BF, Aksamit AJ, Keegan M, et al. (2004) Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology 62(7): 1177–1182.
- Keime-Guibert F, Graus F, Broët P, Reñé R, Molinuevo JL, et al. (1999) Clinical outcome of patients with anti-Hu-associated encephalomyelitis after treatment of the tumor. Neurology 53(8): 1719-1723.
- Britton PN, Dale RC, Blyth CC, Clark JE, Crawford N, et al. (2020) Causes and Clinical Features of Childhood Encephalitis: A Multicenter, Prospective Cohort Study. Clinical Infectious Diseases 70(12): 2517–26.
- Mailles A, De Broucker T, Costanzo P, Martinez-Almoyna L, Vaillant V, et al. (2012) Long-term Outcome of Patients Presenting With Acute Infectious Encephalitis of Various Causes in France. Clinical Infectious Diseases 54(10): 1455–1464.
- Mithal LB (2016) The Burden of Pediatric Encephalitis in the United States. Pediatr Neurol Briefs. 30(10): 38.
- Michaeli O, Kassis I, Shachor-Meyouhas Y, Shahar E, Ravid S (2014) Long-term Motor and Cognitive Outcome of Acute Encephalitis. PEDIATRICS 133(3): e546–552.
- Houtrow AJ, Bhandal M, Pratini NR, Davidson L, Neufeld JA (2012) The Rehabilitation of Children with Anti–Nmethyl-D-aspartate–Receptor Encephalitis: A Case Series. American Journal of Physical Medicine & Rehabilitation 91(5): 435-441.
- Howarth RA, Vova J, Blackwell LS (2019) Early Functional Outcomes for Pediatric Patients Diagnosed withAnti-N-Methyl-D-Aspartate Receptor Encephalitis during Inpatient Rehabilitation. Am J Phys Med Rehabil 98(7): 529-535.
- (1993) Guide for the Functional Independence Measure for Children (WeeFIM) of the Uniform Data System for Medical Rehabilitation, Version 4.0—Community/Outpatient. State University of New York at Buffalo, USA.
- Ottenbacher KJ, Msall ME, Lyon NR, Duffy LC, Granger CV, et al. (1997) Interrater agreement and stability of the functional independence measure for children (weefimTM): Use in children with developmental disabilities. Archives of Physical Medicine and Rehabilitation 78(12): 1309–1315.
- Hannigan KF (2008) Teaching Intermittent Self-catheterization to Young Children with Myelodysplasia. Developmental Medicine & Child Neurology 21(3): 365-368.
- Tailor YI, Suskauer SJ, Sepeta LN, Ewen JB, Dematt EJ, et al. (2013) Functional status of children with encephalitis in an inpatient rehabilitation setting: a case series. J Pediatr Rehabil Med 6(3): 163-173.
- Alvarez G, Krentzel A, Vova J, Blackwell L, Howarth R (2021) Pharmacologic Treatment and Early Rehabilitation Outcomes in Pediatric Patients With Anti-NMDA Receptor Encephalitis. Arch Phys Med Rehabil 102(3): 406-412.
- Bagdure D, Custer JW, Rao S, Messacar K, Dominguez S, et al. (2016) Hospitalized Children with
-
Jussely Morfin*, Katherine Thomas, Arshad Ali, Benjamin Dirlikov, Yumi Mitsuya and Thao Duong. Inpatient Rehabilitation Admission Rate and Functional Improvement in Pediatric Patients with Autoimmune Encephalitis. Arch Neurol & Neurosci. 13(1): 2022. ANN.MS.ID.000805.
-
Autoimmune encephalitis; Functional outcomes; Functional independence measure; Pediatric rehabilitation; Children and adolescents, Encephalomyelitis, Deficit/Hyperactive Disorder, Immunoglobulin,
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






