 Mini Review
Mini Review
Good Response to Thiamine in Bilateral Thalamic Infarction Simulating a Wernicke Syndrome: Does it Has a Role in Acute Stroke?
Vargas Cañas, Alberto1,2*, Velásquez Mauricio1, Martínez Alejandro1and Valencia Javiera3
1Neurology Unit, Hospital Luis Tisné, Santiago, Chile
2Medicine School, University of Los Andes, Santiago, Chile
3Internal Medicine Fellowship, University of Los Andes, Santiago, Chile
Vargas-Cañas Alberto, Neurology Unit, Hospital Luis Tisné, Santiago, Chile.
Received Date: May 17, 2021; Published Date: June 24, 2021
The classic clinical triad of Bilateral Thalamic Infarction is consciousness compromise, ocular motility disturbances, and cognitive deterioration; and would be an obligatory differential diagnosis of Wernicke´s Syndrome, which usually has, as clinical findings, altered mental status, ataxic gait and ophtalmoplegia. While Wernicke´s Syndrome is frequently associated with alcohol intake, it is known that there are some cases not related to alcohol consumption, these subtypes are called atypical non-alcoholic Wernicke´s Syndrome and are provoked by malnutrition as their most important etiology. Clinical case: female patient, admitted to our hospital with sudden installation of postural instability, tendency to drowsiness, nausea, dysarthria and provoked-type confabulation, initially diagnosed as an atypical non-alcoholic Wernicke´s Syndrome, with a first cerebral tomography without alterations and good response to thiamine infusion. In a second image study, a Bilateral Thalamic Infarction was evidenced. According to many reviews, the use of thiamine in acute stroke is not useful; and would be recommended in the neuro-rehabilitation phase. The clinical regression of symptoms and signs, in our patient, would be the natural history of some series of patients with Bilateral Thalamic Infarction described in the literature; but, we propose that it could also be explained in a bilateral thalamic dysfunction previously described in Wernicke´s Syndrome, and its fast response to thiamine use; so it would be interesting publishing more clinical reports or trials using thiamine in this specific type of stroke.
Keywords:Bilateral Thalamic Infarction; Wernicke Syndrome; Thiamine; Acute Stroke
Introduction
The Wernicke Syndrome (WS) is a serious and potentially lethal neuropsychiatric disease [1], of acute onset, caused by a deficiency of vitamin B1(thiamine); with an estimated prevalence between 0.4% and 2.8% [1,2,4]. It is characterized by ophtalmoplegia, ataxia and compromised consciousness [1-3]; this classic triad is observed only in 16%-33% of patients; and is more frequently observed in alcoholics patients [2]. About 19% of patients do not have any of these symptoms initially. The most frequent symptom is the alteration of mental state, that is present in 34%-82% of all cases, and is explained by the compromise of the reticular system at thalamic nuclei or mammillary bodies level; and is followed by the alteration of the ocular motility that is due to a lesion of the pontine tegmentum [2]. On the other hand, the ataxia can be explained by the compromise of the cerebellar vermis and vestibular alteration [2]. The diagnosis of WE is clinical, so it requires a high degree of suspicion [1].
Alcoholism is described as the main cause, but only accounts for 50% of cases. Non-alcoholic WS cases have atypical symptoms, leading to late or under-diagnosis [1]; and is mainly related to malnutrition, chemotherapy, hyperemesis gravidarum, gastrointestinal surgeries, dialysis, parenteral nutrition, thyroid pathology, and psychiatric disorders such as anorexia. The mainstay of treatment is the soon administration of thiamine [1]; and, since diagnostic confirmation of WS can often be difficult and ever late, when WS is suspected, treatment should be started early [2]. This consideration is due to the fact that the administration of thiamine is safe, inexpensive, and the prompt supplementation prevents the progression of WS to irreversible deficits, leading a rapid improvement of symptoms, especially alterations in mental status, and ocular motility [1,2]. On WS, the cerebral tomography (CT) can show hypodense areas in the periaqueductal gray matter, and the medial region of the thalamus; but in most cases, there are no alterations in the acute phase of WS. On the other hand, magnetic resonance imaging (MRI) is highly specific and has great value in the diagnostic confirmation of WS, showing a symmetric and bilateral hyperintensity at the paraventricular level of the thalamus, hypothalamus, mammillary bodies, periaqueductal region and floor of the fourth ventricle [1,2].
One of the rarest types of stroke is bilateral thalamic infarction (BTI); with an estimated incidence of 0.6% of all strokes, and between 22%-35% of all thalamic strokes [5]; and is associated with an anatomical variant in the cerebral posterior irrigation, named Percheron´s artery [5,6]; and has some usual symptoms like depression of wakefulness, amnesia, confabulation, aphasia (if lesion is in the dominant hemisphere), apathy and agitation, among others [6]; an also paralysis of ocular motility [5]. The natural evolution of BTI is very diverse; showing cases of spontaneous resolution of symptoms in hours to days [5], secondary dementia [7,8] and death [5].
Case Report
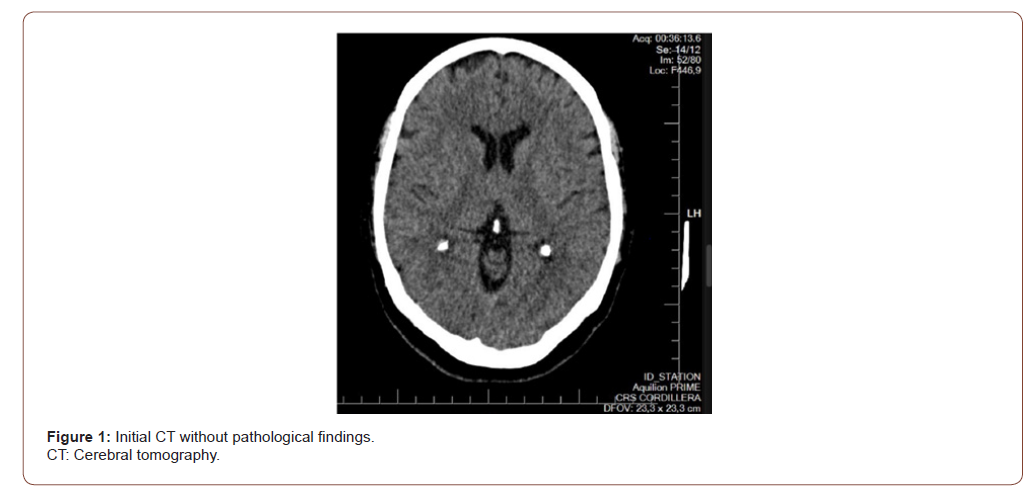
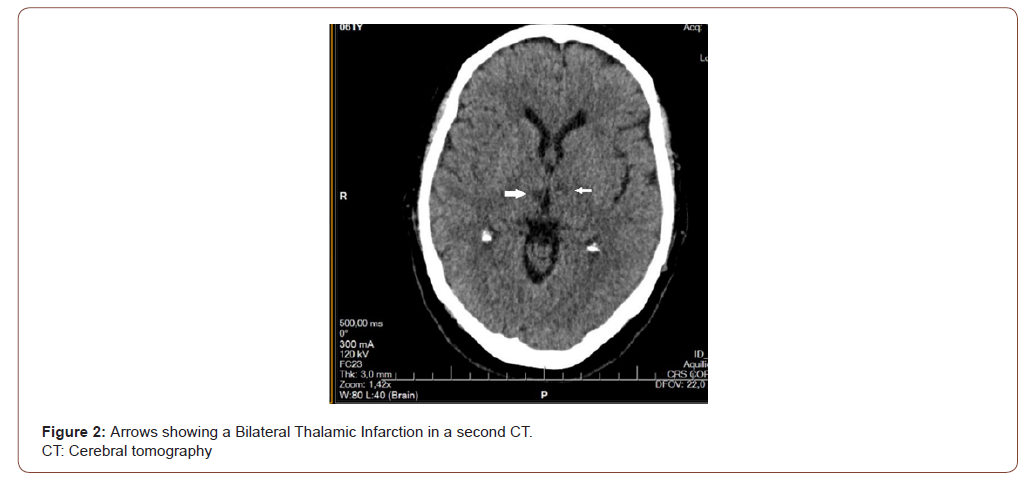
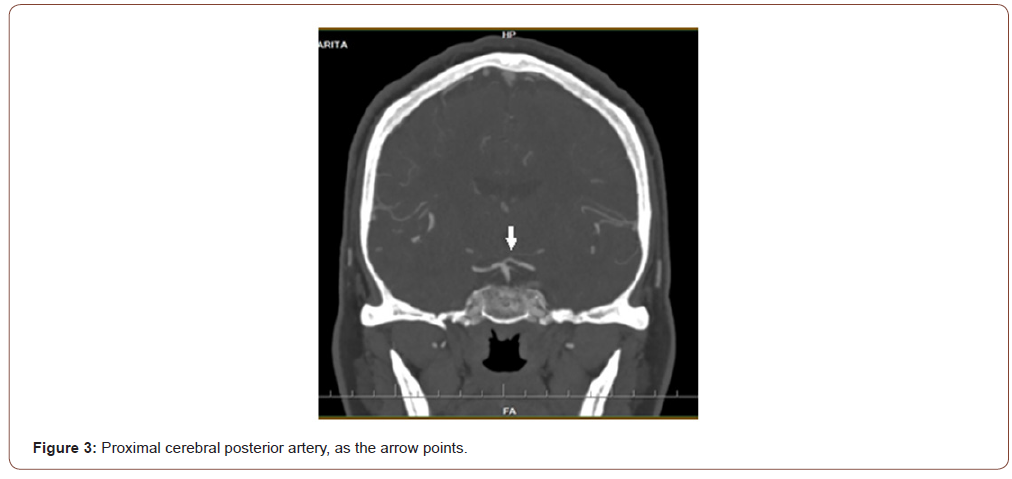
Female patient, 61 years old, active smoker, with diagnosis of chronic depression, and Rankin Scale at 0. The patient consults to our hospital due to 24 hours of sudden onset of postural instability, tendency to drowsiness, nausea and dysarthria; without history of headache or fever. At emergency room (ER), the blood pressure, glycemic value, and other biochemical parameters were normal. An electrocardiogram was performed that did not show alterations. The neurological examination is described like alertness with tendency to drowsiness, unable to invert automatic series; dysarthria, provoked type confabulation, pupillary anisocoria reactive to light, limitation of vertical gaze without nystagmus; and bilateral dysmetria of great intensity to right side. At ER, a therapeutic test with flumazenil was made, because a benzodiazepine poisoning was suspected; but without response. A CT, shown in Figure 1, and a lumbar puncture were made, all without pathological findings. Due to suspicion of probable WE or non-alcoholic cause, thiamine is started at dose of 1500mg / day with good response at 24 hours; and was maintained for 5 days. Because the unusual and rapid onset of clinical symptoms, a second CT was made showing a subacute bilateral thalamic ischemic lesion, as seen in Figure 2; associated to a proximal left cerebral posterior artery stenosis as is evidenced in Figure 3. The patient´s mental status, speech, dysmetria and ocular motility evolves to total regression. There were no complications during hospitalization.
Discussion
As previously stated, WS may or may not be associated with alcohol (OH) intake [1] and should be suspected in patients with rapid and progressive onset of altered mental status, ocular motility palsy and ataxia [2]; and usually in that clinical sequence. This triad of symptoms was present in our patient, and although there was no history of OH ingestion, a WS was suspected, especially when an easy provoked-type confabulation was evidenced in the patient. This last finding, the confabulation, has been associated with WS since it was initially described by Korsakoff in 1889, in amnesic alcoholic patients; however, it has been described also in other conditions such as anterior communicating artery rupture, traumatic brain injury, Alzheimer´s disease and brain tumor [9].
It is well recognized that early onset of thiamine administration in WS is recommended because it has low cost, general safety, rapid improvement in symptoms, and prevention of progression to irreversible brain damage [1]. Actually there is no consensus on the therapeutic dose, but the recommendation of the European Federation of Neurological Societes (EFNS) is for intravenous infusion of thiamine, 200mg diluted in 100cc of 5% dextrose or normal saline; in approximately 30 minutes [1]; however, according to our experience, it has to be supplemented for a minimum of 5 days. This treatment was done in this clinical case, showing improvement in 24 hours. The good evolution of our patient in response to thiamine use, the absence of alcohol intake, and the early pupillary comprise in this clinical case did caught our Citation: Vargas Cañas, Alberto, Velásquez Mauricio, Martínez Alejandro, Valencia Javiera. Good Response to Thiamine in Bilateral Thalamic Infarction Simulating a Wernicke Syndrome: Does it Has a Role in Acute Stroke?. Arch Neurol & Neurosci. 10(4): 2021. ANN.MS.ID.000742. DOI: 10.33552/ANN.2021.10.000742. Page 4 of 4 attention, and it made us suspect another condition like BTI in this case. Although thiamine is not recommended in acute stroke, there is some evidence of usefulness in post-cardiorespiratory arrest encephalopathy [10], and there are also reports of benefits in the neuro-rehabilitation stages of stroke, by improving post-stroke fatigue of this type of conditions [11].
On the other hand, although hypodense areas in the periaqueductal gray matter, and the medial region of the thalamus has been described in CT of patients with WS [14], how was found in our patient, segmental stenosis of the posterior cerebral artery is not a finding as it was evidenced in this clinical case. It is possible that the improvement of our patient may be due the natural evolution of some patients with BTI, described as 3 to 24 hours [5]; but we theorize that it could be related to the action mechanism of thiamine in some WS cases reports, in which dysfunction in bilateral thalamic perfusion had been found [12-15], just like patients with BTI.
Conclusion
The differential diagnosis of WS, especially in cases not associated to OH intake, must be broad and rapid for an early onset of thiamine; and among these diagnoses, BTI should be included due to the similarity in the clinical characteristics. A possible way to make the differential diagnosis is the more acute onset and the pupillary alterations in BTI. Finally, although the thiamine is not useful in the acute management of stroke –in BTI– for having some similarity with WS in the pathophysiology, it could be possible that it has a role in this subtype of stroke. It would be recommended to increase the report of this type of case with thiamine supplementation in acute BTI.
Acknowledgement
To our patients; as they are the mainstay of our work. To our families; as their support and love is our strength. To Alvaro Vargas Oñate; as his correct knowledge of the English language facilitates our work.
Conflict of Interest
No conflict of interest.
References
- Singla S, Singla R (2016) Stroke in India: Bio-socioeconomic determinants. J Soc Health Diabetes 04(02): 071-6.
- Kamalakannan S, Gudlavalleti ASV, Gudlavalleti VSM, Goenka S, Kuper H (2017) Incidence & prevalence of stroke in India: A systematic review. Indian J Med Res 146(2): 175-185.
- Pandian JD, Sudhan P (2013) Stroke Epidemiology and Stroke Care Services in India. J Stroke. 15(3): 128-134.
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, et al. (2013) An Updated Definition of Stroke for the 21st Century: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 44(7): 2064-2089.
- NINDS Post-Stroke Rehabilitation [Internet]. Available from: https://www.stroke.nih.gov/materials/rehabilitation.htm
- Sunderland A (2000) Recovery of ipsilateral dexterity after stroke. Stroke 31(2): 430-433.
- Boukadida A, Piotte F, Dehail P, Nadeau S (2015) Determinants of sit-to-stand tasks in individuals with hemiparesis post stroke: A review. Ann Phys Rehabil Med 58(3): 167-172.
- Warlow CP, Dennis MS, Van Gijn J, Hankey G, Barnett HJM, et al. (2001) Stroke- A practical guide to management. 2nd ed. United Kingdom: Blackwell.
- Fahey M, Crayton E, Wolfe C, Douiri A (2018) Clinical prediction models for mortality and functional outcome following ischemic stroke: A systematic review and meta-analysis. PLOS ONE 13(1): e0185402.
- Tempest S, McIntyre A (2006) Using the ICF to clarify team roles and demonstrate clinical reasoning in stroke rehabilitation. Disabil Rehabil 28(10): 663-667.
- Silva SM, Corrêa FI, Faria CD, Buchalla CM, Silva PF, et al. (2015) Evaluation of poststroke functionality based on the International Classification of Functioning, Disability, and Health: a proposal for use of assessment tools. J Phys Ther Sci 27(6): 1665- 1670.
- ICF Definition- WHO. https://www.who.int/classifications/icf/icfbeginnersguide.pdf?ua=1
- Sykes C (2006) Health Classifications 1 - An introduction to the ICF. WCPT Keynotes. World Confederation for Physical Therapy.
- Brasure M, Lamberty GJ, Sayer NA, Nelson N, Macdonald R, et al. (2012) Multidisciplinary Post-acute Rehabilitation for Moderate to Severe Traumatic Brain Injury in Adults [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); (Comparative Effectiveness Reviews, No. 72.).
- Hislop H, Montgomery J (2007) Daniels and Worthingham’s Muscle Testing: Techniques of Manual Examination, 8th ed. W.B. Saunders St. Louis.
- Lunar FRM, Gorgon EJR, Lazaro RT (2017) Clinimetrics of the Upright Motor Control Test in chronic stroke. Brain Behav 7: e00826.
- Janssen WG (2008) The sit-to-stand movement recovery after stroke and objective assessment, Ph.D. dissertation Erasmus Medical Center Erasmus University Rotterdam The Netherlands.
- Janssen WG, Bussmann HB, Stam HJ (2002) Determinants of the sit-to-stand movement: a review. Physical Therapy 82(9): 866-879.
- Oh D (2013) Community Ambulation: Clinical Criteria for Therapists’ Reasoning and Decision-making in Stroke Rehabilitation. International Jrnl of Physical Med and Rehab 1(4): 2329-9096.
- Adegoke BO, Olaniyi O, Akosile CO (2012) Weight bearing asymmetry and functional ambulation performance in stroke survivors. Glob J Health Sci 4(2): 87-94.
- Perry J, Garrett M, Gronley JK, Mulroy SJ (1995) Classification of walking handicap in the stroke population. Stroke 26(6): 982-989.
- Shumway-Cook A, Wollacott MH (1995) Motor Control: Theory and Practical Applications. Baltimore, Md: Williams and Wilkins Inc.
- MMSE guidelines. https://www.ncbi.nlm.nih.gov/projects/gap/cgibin/ GetPdf.cgi?id=phd001525.
- Mao YR, Wu XQ, Zhao JL, Le L, Xiu QW, et al. (2018) The Crucial Changes of Sit to Stand Phases in Subacute Stroke Survivors Identified by Movement Decomposition Analysis. Front Neurol 9: 185.
- Wagatsuma M, Kim T, Sitagata P, Lee E, Vrongistinos K, et al. (2019) The biomechanical investigation of the relationship between balance and muscular strength in people with chronic stroke: a pilot cross-sectional study. Top Stroke Rehabil 26(3): 173-179.
- Berger RA, Riley PO, Mann RW, Hodge WA (2019) Total body dynamics in ascending stairs and rising from a chair following total knee arthroplasty. Trans Orthop Res Soc 13: 542.
- de Haart M, Geurts AC, Dault MC, Nienhuis B, Duysens J (2005) Restoration of weight shifting capacity in patients with postacute stroke: a rehabilitation cohort study. Arch Phys Med Rehabil 86(4): 755-762.
- Dettmann MA, Linder MT, Sepic SB (1987) Relationships among walking performance, postural stability, and functional assessments of the hemiplegic patient. Am J Phys Med 66: 77-90.
- Lomaglio M, Eng J (2005) Muscle strength and weight-bearing symmetry relate to sit-to-stand performance in individuals with stroke. Gait and Posture 22(2): 126-131.
- Bohannon RW (2007) Muscle strength and muscle training after stroke. J Rehabil Med 39(1):14-20.
- Xu J, Ejaz N, Hertler B, Widmer M, Kitago T, et al. (2017) Separable systems for recovery of finger strength and control after stroke. J Neurophysiol 118(2): 1151- 1163.
- Geyh S, Cieza A, Schouten J, Dickson H, Frommelt P, et al. (2004) ICF Core Sets for stroke. J Rehabil Med 44: 135-141.
- Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A (2002) Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci 57(8): M539-M543.
- Pinheiro M, Polese JC, Machado G, Scianni AA, Hirochi TC, et al. (2010) Balance analysis during the sit-to-stand movement of chronic hemiparetic individuals based upon their functional levels. Man Ther Posturology Rehabil J 12: 260-264
- Akulwar IS (2019) Can Quantitative Balance Measures Discriminate between Functional Ambulation Categories in Chronic Stroke Survivors? Physiother Rehabil 4: 178.
- Ju S (2020) Correlation between lower limb muscle asymmetry during the sit-to-stand task and spatiotemporal gait asymmetry in subjects with stroke. J Exerc Rehabil 16(1): 64-68.
- Lee G, An S, Lee Y, Park DS (2016) Clinical measures as valid predictors and discriminators of the level of community ambulation of hemiparetic stroke survivors. J Phys Ther Sci 28(8): 2184-2189.
- Danielsson A, Meirelles C, Willen C, Sunnerhagen KS (2014) Physical activity in community-dwelling stroke survivors and a healthy population is not explained by motor function only. PM R 6(2): 139-145.
- Akbari A, Karimi H (2006) The Relationship Between Lower-extremity Muscle Strength and Functional Performance in Hemiparetic Patients. Journal of Medical Sciences 6: 327-331.
- Moriello C, Finch L, Mayo NE (2011) Relationship between muscle strength and functional walking capacity among people with stroke. J Rehabil Res Dev 48(3): 267-275.
- Nadeau S, Arsenault AB, Gravel D, Bourbonnais D (1999) Analysis of the clinical factors determining natural and maximal gait speeds in adults with a stroke. Am J Phys Med Rehabil 78(2): 123-130.
- Eng JJ, Tang PF (2007) Gait training strategies to optimize walking ability in people with stroke: a synthesis of the evidence. Expert Rev Neurother 7(10): 1417-1436.
-
Vargas Cañas, Alberto, Velásquez Mauricio, Martínez Alejandro, Valencia Javiera. Good Response to Thiamine in Bilateral Thalamic Infarction Simulating a Wernicke Syndrome: Does it Has a Role in Acute Stroke?. Arch Neurol & Neurosci. 10(4): 2021. ANN.MS.ID.000742.
-
ICF; Stroke; UMCT; STS; FAC; Impairments; Participation; Activity limitation, Activities of daily living; International Classification of Functioning, Disability and Health; Sit to Stand; Upright motor control test; Functional Ambulation Capacity.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






