 Research Article
Research Article
Comparative Role of Paroxatine and Clomipramine on Anxiety in Mice Recovering from Stress
Mfem CC1, Nyoro IK1 and Seriki SA*2
1Department of Human Physiology, University of Calabar, Nigeria
2Department of Human Physiology, Edo University, Nigeria
Seriki SA, Department of Human Physiology, College of Medical Sciences, Edo University, Iyamho, Nigeria.
Received Date: August 08, 2019; Published Date: August 21, 2019
Abstract
Stress is the body’s reaction to any change that requires an adjustment or response. The body reacts to these changes with physical, mental, and emotional responses. It is the body’s reaction to harmful situations whether they’re real or perceived. When stressed, a chemical reaction occurs in the body that allows it to act in a way to prevent injury. This reaction is known as “fight-or-flight,” or the stress response. During stress response, heart rate increases, breathing quickens, muscles tighten, and blood pressure rises. The present study investigated behavioral pattern between stress-induced mice and stressed mice treated with antidepressants (paroxatine and clomipramine) using open field apparatus and elevated-plus maze test. Twenty eight (28) Swiss Mice were divided equally into four groups i.e. Group one (control), Group two (stressed), Group three (stressed mice treated with paroxatine) and Group four (stressed mice treated with clomipramine). Animals in group-one were given normal feed and water only. Group two animals were also given normal rat chew and water and subjected to prolonged stress by social isolation, deprivation, confinement to small cages and exposure to bright light at night for a period of five weeks. Animals in group three were administered with paroxatine at a dose of 10mg/kg body weight orally for two (2) weeks after they were stressed for five-week, while animals in the group four were administered with clomipramine at the same dose orally for two (2) weeks after subjecting them to the same measure and duration of stress like those of group three. The treated groups were also allowed access to water and normal rat feeds throughout the experiment period. The four groups were then subjected to tests in both open field and elevated-plus maze apparatus. The results showed increased anxiety in the stressed mice compared to control. The groups stressed and later treated with paroxatine and clomipramine showed significantly reduced anxiety (P<0.05) compared to stressed mice. The group stressed and later treated with paroxatine showed reduced anxiety significantly compared to the group stressed and later treated with clomipramine (P<0.05). These were determined by grooming frequency and duration, rearing frequency as well as closed arm duration and head dip frequency in the EPM. Paroxatine and clomipramine administration resulted in significant improvement from anxiety after stress. Paroxatine caused a more significant improvement from anxiety than clomipramine in mice recovery from stress when the two agents were compared.
Keywords:Anxiety; Stress; Paroxatine; Clomipramine
Introduction
Stress has become undoubtedly an integral part of human life. Stressful events have a damaging effect on normal physiological functions leading to a variety of diseases. Many of the diseases of the modern life like hypertension, diabetes, behavioral disorders have been suggested as some of the many deteriorating effects of stress [1]. Stress is an important factor of depression that causes the changes in various body systems. Animal health including human has been shown to be affected by the stressful events of life including situation which alters cognition, learning, memory and emotional responses, causing mental disorders like depression and anxiety. Depression is a state of low mood and aversion to activity that can affect a person’s thoughts, feelings, behavior and sense of wellbeing [2]. However, many of these effects are mediated by stressinduced neurochemical and hormonal abnormalities that are often associated with oxidative stress [3]. People with depressed mood may be notably sad, anxious, or empty; they may also feel hopeless, helpless, dejected or worthless. Other symptoms expressed may include senses of guilt, irritability or anger. These individuals may express a further feeling of shamefulness or an expressed restlessness. They may notably lose interest in activities that they once considered pleasurable to family and friends or otherwise experience either a loss of appetite or overeating. Experiencing problems like concentrating, remembering general facts or details, otherwise making decisions or experiencing relationship difficulties may also be notable factors in these individuals’ depression and may also lead to their attempting or actually committing suicide [4]. In addition to all the aforementioned factors, actions committed by siblings of these individuals may also contribute to the decisionmaking in individuals experiencing depression or attempting to take their own lives [4]. Expressed insomnia, excessive sleeping, fatigue, pains, digestive problems and a reduced energy may also be present in individuals experiencing depression. A depressed mood is a feature of some psychiatric syndromes such as major depressive disorder and dysthymia, but it may also be a normal temporary reaction to life events such as bereavement, a symptom of some bodily ailments or a side effect of some drugs and medical treatments.
Antidepressants are drugs used for the treatment of major depressive disorder and other conditions, including dysthymia, anxiety disorders, obsessive compulsive disorder, eating disorders, chronic pain, neuropathic pain and, in some cases, dysmenorrheal, snoring, migraine, attention-deficit hyperactivity disorder, addiction, dependence, and sleep disorders [5]. The most important classes of antidepressants are the selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (TeCAs), and noradrenergic and specific serotonergic antidepressant [6]. Antidepressants are recommended by the National Institute for Health and Care Excellence (NICE) as a firstline treatment of several depressions and for the treatment of mildto- moderate depression that persists after conservative measures such as cognitive therapy. There has been controversy regarding the efficacy of antidepressants in treating depression depending on its severity and duration. The 2008 meta-analysis combined 35 clinical trials submitted to the Food and Drug Administration (FDA) before licensing of four newer antidepressants (including the SSRIs paroxatine and flouxatine, the non-SSRI antidepressant nefazodone, and the SNRI venlafaxine). The authors attributed the relationship between severity and efficacy to a reduction of the placebo effect in severely depressed patients, rather than an increase in the effect of the medication. SSRIs are recommended by the National Institute for Health and Care Excellence (NICE) for the treatment of generalized anxiety disorder (GAD) that has failed to respond to conservative measures such as education and self-help activities [7]. Among the SSRIs, paroxatine has the highest specificity for serotonin. Paroxatine affects chemicals in the brain that may be unbalanced in people with depression, anxiety, or other disorders. It is effective in depression that has proved resistant to other antidepressants and in depression complicated by anxiety [8].
Paroxatine is used to treat depression, obsessive-compulsive disorder, anxiety disorders, post-traumatic stress disorder (PTSD), and premenstrual dysphonic disorder (PMDD).Common side effects of paroxatine may include: vision changes; weakness, drowsiness, dizziness; sweating, anxiety, shaking; sleep problems (insomnia); loss of appetite, constipation; dry mouth, yawning; or decreased sex drive, impotence, or difficulty having an orgasm. Other antidepressants used in the treatment of obsessive-compulsive disorder, major depressive disorder, panic disorder, are the tricyclic antidepressants (TCAs). Clomipramine belongs to this group and is a highly selective inhibitor of serotonin reuptake [9]. It is also an antagonist/inverse agonist at the histamine H1 receptor, the muscarinic acetylcholine receptors and the α1 adrenergic receptor. These last three actions likely contribute to its adverse effects [9].
The nervous system is a coordinating system that controls all the activities of the body. It receives millions of bits of information from the different sensory organs and then integrates all these to determine the responses to be made by the body. It is a comumunication network that allows an individual to interact appropriately with the environment. The nervous system consists of sensory and motor divisions. The sensory division initiates most of its activities emanating from sensory receptors whether visual, auditory, tactile on the surface of the body or other kinds of receptors. The motor division controls the various body activities like contraction of skeletal muscles, smooth muscles in the internal organs and secretion of both endocrine and exocrine organs [10]. These are collectively called the motor functions of the nervous system. Primarily, the nervous system is divided into two parts namely, the central nervous system and the peripheral nervous system. The central nervous system includes the brain and spinal cord. It is formed by neurons and the supporting cells called neuroglia. The functions of the central nervous system among others include organizing reflexes and other behavioural responses responsible for cognition, learning and memory and plans and executes voluntary movements. Memory refers to the storage mechanisms for what is learned, learning and memory are special forms of information processing that permit behaviour to change appropriately in response to environmental challenges based on past experiences. The peripheral nervous system is formed by the neurons and their processes present in all regions of the body. These consist of cranial nerves from the brain and spinal nerves from the spinal cord. It is further subdivided into the somatic nervous system and autonomic nervous system. The somatic nervous system controls the movements of the body by acting on the skeletal muscles while the autonomic nervous system is concerned with regulation of visceral or vegetative functions [10]. Behaviour consists of the totality of the organism’s responses to its environment.
Aim of the study
The aim of this study is to compare the effect of two antidepressants (Paroxetine and Clomipramine) on anxiety in stressed mice.
Materials and Methods
Materials
The following materials were used for the various experiments carried out: methylated spirit, weighing balance, plastic bowl, syringe and needle, distilled water, cotton wool, Toilet paper, open field maze, Elevated plus maze, Marker pen, cages, feeding troughs, milk, spatula, masking tape, stop watches, sawdust beddings.
Experimental animals and drugs
The experimental animals used in this study were 28 Swiss mice, obtained from Department of Pharmacology, University of Calabar, Calabar, Nigeria with body weight between 180gto 250g specifically bred for experimental purposes. The mice were divided into four groups of seven each.
• Group 1: The control (non-stressed).
• Group 2: Stressed group (stressed but not treated).
• Group 3: Stressed + paroxatine group.
• Group 4: Stressed + clomipramine group.
Group 1 was not exposed to any form of stress. Group 2 were stressed but not treated. Group 3 were stressed but treated with oral paroxatine (10mg/kg body weight) daily for 2 weeks. Group 4 were stressed but treated with oral clomipramine (10mg/kg body weight) daily for 2 weeks. All animals were allowed access to feed and water.
Drug administration protocol
The doses of drugs, the duration of the administration were done according to previous studies as reported by [11]. All drugs were dissolved in distilled water.
Administration of paroxatine and clomipramine
A 20mg and a 10mg capsule of paroxatine and clomipramine was dissolved in 20ml and 10ml of distilled water (using a stirrer) to form a stock solution of 0.02mg/20ml and 0.01/10ml respectively. Each of the solution was administered at the dose of 10mg/kg body weight orally, once daily for two weeks.
Stress regimen
This procedure was done through social isolation (prolonged social deprivation), confinement to small cages and exposure to bright light at night for the period of five weeks [12] (Matsumoto et al, 2005).
Statistical Analysis
Values for the result are expressed as mean ± SEM. The statistical analysis was done using the analysis of variance (ANOVA) and the post/hoc Newmann Keul’s test. The computer software was Microsoft excel and SPSS for window.
Differences between means were tested at 0.05 level of significance.
Apparatus and experimental protocol
The open field maze: The large open field (72 × 72 cm, with 36cm high walls) constructed of plywood was used following the protocol of [13]. Each trial was recorded using a video cassette recorder (VCR) connected to a camcorder. This was for rescoring of behaviour.
Experimental procedure in the open field maze:Mice were carried to the neurobehaviour laboratory in their home cages from the animal house and were handled by the base of their tails at all times. Each mouse of the four groups was exposed to the open field maze by placing it in the centre square of the maze and allowed to explore the apparatus for 5 minutes. The mouse behavior was scored within this period and the mouse returned to its home cage while the open field was cleaned with 70% ethyl alcohol and then allowed to dry between tests. This was to eliminate olfactory cue.
Behaviour scores in the open field
The behaviours scored included;
1. Line crossing: Number of times the mouse crossed a line drawn on the floor with all its four paws.
2. Rearing: Frequency with which the animal stands on its hind legs or leans against the wall with front paws.
3. Center square duration: Amount of time the mice spent in the central square.
4. Center square entries: Frequency with which the mouse enters the centre square with all four paws.
5. Grooming frequency: the number of times the animal spent licking or scratching itself while stationary.
6. Grooming duration: The period of time the animal spent licking or scratching itself while stationary.
7. Urination: Number of puddles or streaks of urine.
8. Defecation: Number of fecalboli produced.[13]
Elevated plus maze
Experimental procedure in the elevated plus maze
The mouse was picked by the base of its tail from the home cage and placed in the center square between the open and closed arms facing an open arm. Each mouse was allowed to explore the maze for 5 minutes and the behaviours scored within this period and then returned to its home cage. The apparatus was cleaned with 70% ethyl alcohol and then allowed to dry between tests in order to eliminate olfactory cue.
Behaviour scores in the elevated plus maze
1. Open arm entries: Frequency with which the animal entered the open arms with all four paws.
2. Closed arm entries: Frequency with which the mouse entered the closed arm with all four paws.
3. Open arm duration; length of time the animal spent in the open arms.
4. Closed arm duration: Length of time the animal spent in the open arms.
5. Head dipping: Frequency with which the animal lowered its head over the sides of the open arms toward the floor.
6. Rearing: Frequency with which the animal stands on its hind legs or leans against the wall with front paws.
7. Grooming (Frequency and duration): The number and period of times the animal spends licking or scratching itself while stationary.
8. Urination: Number of puddles or streaks of urine.
9. Defecation: Number of fecalboli produced. [13-16]
Results
Frequency of grooming in the open field for control, stressed, stressed+paroxatine, and stressed+clomipramine groups
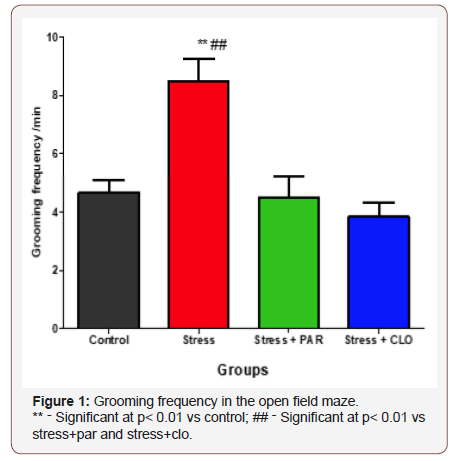
The grooming frequency in the open field for control, stressed, stressed+paroxatine and stressed+clomipramine were, 4.7 ± 0.42, 8.5 ± 0.76, 4.5 ± 0.72, and 3.8 ± 0.48 respectively.
There was significant increase (P<0.01) in stressed group compared to control, stressed+paroxatine, stressed+clomipramine groups (P<0.01). The results showed no significant difference between stressed+paroxatine and stressed+clomipramine groups, although there was a significant decrease (P<0.01) in both treated groups compared with the stressed group (Figure 1).
Grooming duration in the open field for control, Stressed, stressed+paroxatine, and stressed+clomipramine groups
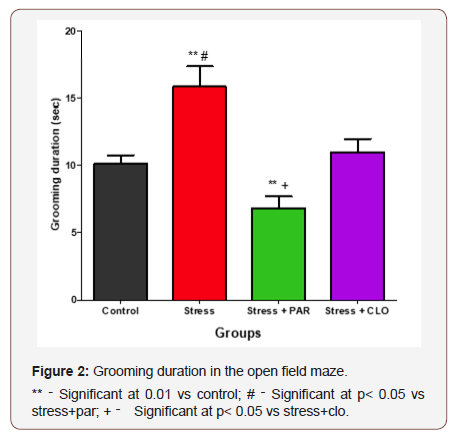
The grooming duration in the open field for control, stressed, stressed+paroxatine, and stressed+clomipramine group of mice were, 10 ± 0.6s,16± 1.5s,6.8 ± 0.87s and 11 ± 0.97s respectively. The stressed group showed a significant increase (p<0.05) compared to control, stressed+paroxatine, and stressed+clomipramine group. It was also observed that stressed mice treated with paroxatine spent lesser time grooming compared with stressed mice treated with clomipramine (P<0.05) (Figure 2).
Centre square entry frequency in the open field for control, stressed, stressed+paroxatine and stressed+clomipramine group
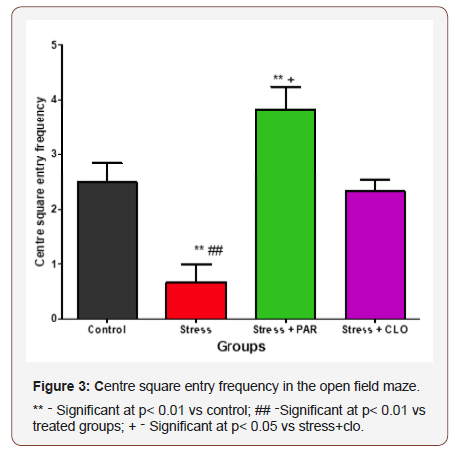
The Centre Square Entry (CSE)frequency for the control, stressed, stressed+paroxatine, and stressed+clomipraminegroup of mice were 2.5 ± 0.34, 0.67 ± 0.33, 3.8 ± 0.40, and 2.3 ± 0.21 respectively. The mice in the stressed group showed a significantly lower (p<0.01) CSE compared to mice in the control, stressed+paroxatine and stressed+clomipramine groups. The stressed+paroxatine group showed a significant increase (p<0.01) in CSE compared with control, but there was no significant difference in stressed+clomipramine group compared with control. Stressed+paroxatine showed a significant increase (p< 0.05) compared to stressed+clomipramine (Figure 3).
CSE duration in the open field for control, stressed, stressed+paroxatine, stressed+clomipramine groups
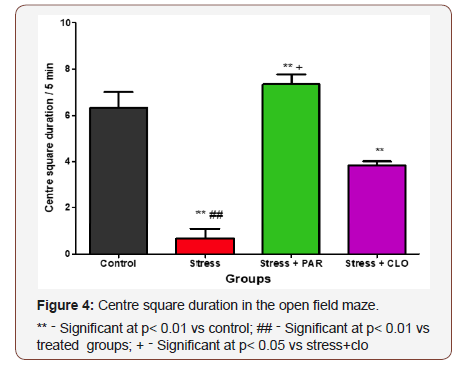
The CSE duration in the open field for control, stressed, stressed+paroxatine and stressed+clomipramine group of mice were6.3 ± 0.67s,0.67 ± 0.42s, 7.3 ± 0.42s, and 3.8 ± 0.17s respectively.
The stressed group was significantly lower (p< 0.01) when compared to control, stressed+paroxatine, and stressed+clomipramine groups.
However, stressed+paroxatine showed asignificant increase in CSE duration compared with control (p< 0.01) and stressed+clomipramine group (P<0.05) (Figure 4).
Open arm entry frequency in the EPM for control, stressed, stressed+paroxetine and stressed+clomipramine group
The open arm entry frequency in the EPM for control, stressed, stressed+paroxatine and stressed+clomipramine groups of mice were,7.0 ± 0.77, 2.2 ± 0.48, 8.0 ± 0.45, and 5.3 ± 0.49 respectively. The stressed group had significantly lower (p<0.01) values of open arm entry frequency compared to control, as well as the treated groups.
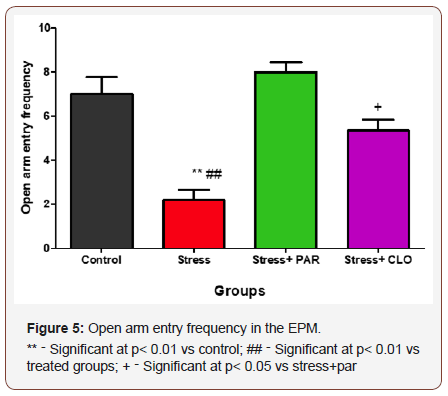
There was no significant difference in OEF in both stressed+paroxatine and stressed+clomipramine groups compared to the control group, but however, there was a significant increase (p<0.05) in OEF in the stressed+paroxatine group compared to stressed+clomipramine group (Figure 5).
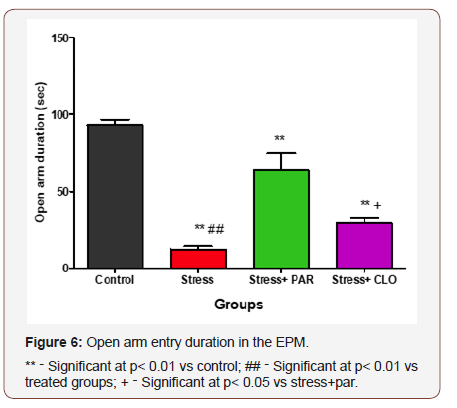
Open arm duration in the EPM for control, stressed, stressed+paroxatine and stressed+clomipramine group
The time spent by the control, stressed, stressed+paroxatine, and stressed+clomipramine group of mice in the open arm were, 93 ± 3.7s, 13 ± 1.9s, 64 ± 11s, and 29 ± 3.3 respectively. Stressed, stressed+paroxatine and stressed+clomipramine groups of mice showed a significant decrease in OAED at P<0.01 compared with control group of mice.
There was a significant increase (p<0.01) in OAED in the stressed+paroxatine group of mice compared with stressed group.
The stressed+paroxatine group showed a significant increase (p<0.05) in OAED compared to stressed+clomipramine group (Figure 6).
Frequency of closed arm entry in the EPM
The closed arm entry frequency of the control, stressed, stressed+paroxatine and stressed+clomipramine groups of mice were, 4.2 ± 0.31, 8.5 ± 0.5, 5.2 ± 0.48, and 5.0 ± 0.37 respectively. The closed arm entry frequency in the stressed group of mice was significantly higher (p<0.01) compared with the control, stressed+paroxatine group, as well as stressed+clomipramine group.
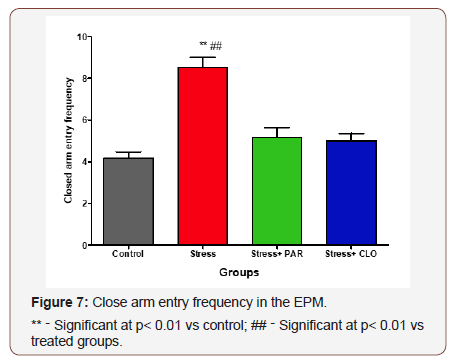
It was observed that, there was no significant difference between the control and the two stressed-treated groups. There was also no significant difference between the two treated groups (Figure 7).
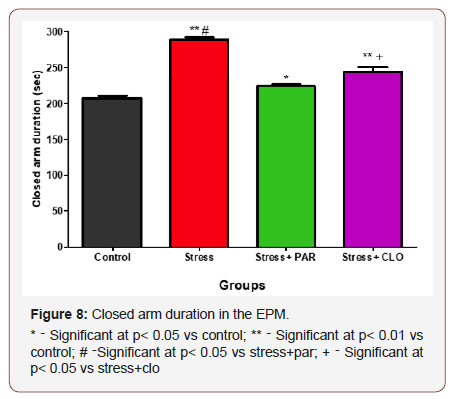
Closed arm duration in the EPM
The closed arm duration in the EPM for control, stressed, stressed+paroxatine and stressed+clomipramine groups of mice were, 207 ± 3.7s, 289 ± 2.6s, 224 ± 2.6s and 244 ± 6.7s respectively. The stressed and stressed+clomipramine groups of mice spent significantly higher time in the closed arm compared to the control at p<0.01. Stressed+paroxatine showed a significant increase in CAD (p< 0.05) compared with control.
There was a significant increase (p< 0.05) in CAD in stressed group compared withstressed+paroxatine. Stressed+clomipramine groups of mice showed a significant increase (p< 0.05) in CAD compared with that of stressed+paroxetine (Figure 8).
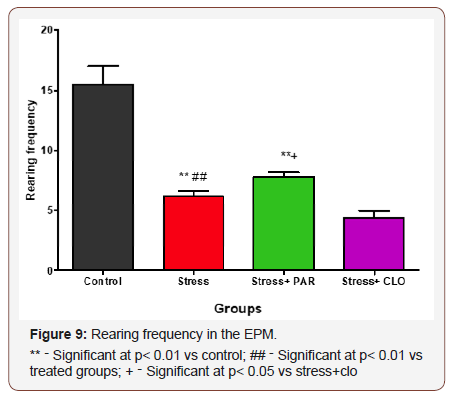
Frequency of rearing in the EPM
The rearing frequency in the EPM for control, stressed, stressed+paroxatine, and stressed+clomipramine group of mice were,16± 1.5, 6.2± 0.48, 7.8±0.40, and 4.3±0.61 respectively. There was a significant decrease in rearing frequency in stressed, stressed+paroxatine, and stressed+clomipramine group of mice compared to the control group at P<0.01.
There was a significant increase (p<0.01) in rearing frequency in stressed+paroxatine group of mice compared to that of stressed group and stressed+clomipramine group at p<0.01 and p<0.05 respectively. (Figure 9)
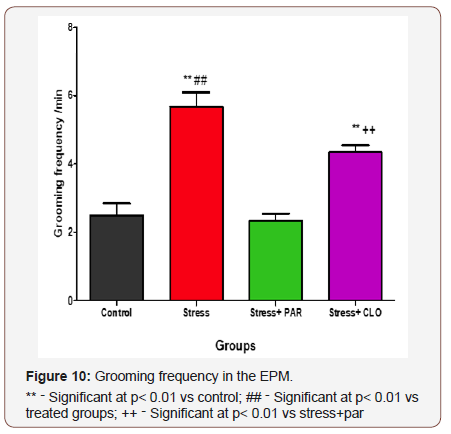
Grooming frequency in the EPM
The grooming frequency for control, stressed, stress+paroxatine, and stress+clomipramine group of mice were, 2.5 ± 0.34, 5.7 ± 0.42, 2.3 ± 0.21 and 4.3 ± 0.21respectively. Stressed group of mice increased significantly (p<0.01) compared to the control, stressed+paroxatine, and stressed+clomipramine groups. Stressed+paroxatine group of mice showed no significant difference compared to control. But however, stressed+clomipramine group of mice was significantly increased (p<0.01) compared to control. Figure 10.
There was also a significant increase (p< 0.01) in the grooming frequency of the stressed-treated with clomipramine group of mice compared to stressed-treated with paroxatine group (Figure 10).
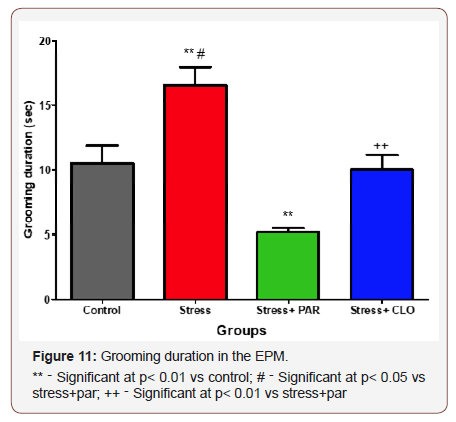
Grooming duration in the EPM
The grooming duration for the control, stressed, stressed+paroxatine, and stressed+clomipramine group of mice were,11± 1.4s, 17 ± 1.4s, 5.2± 0.31s, and 10 ±1.1s respectively. The stressed group of mice showed a significant increase in the grooming time compared to control and stressed+paroxatine groups at p<0.01 and p<0.05 respectively. There was a significant decrease (p<0.01) in the grooming frequency of the stressed+paroxatine group compared to stressed+clomipramine groups (Figure 11).
Frequency of head dip in the EPM
The frequency of head dip for the control, stressed, stressed+paroxatine, and stressed+clomipramine group of mice were, 11 ± 0.49, 3.8 ± 0.48, 7.7 ± 0.49 and 5.3 ± 0.33 respectively. Stressed group of mice showed a significantly decrease (p< 0.01) in head dip compared to control, stressed+paroxatine, as well asstressed+clomipramine group at. (Figure 12)
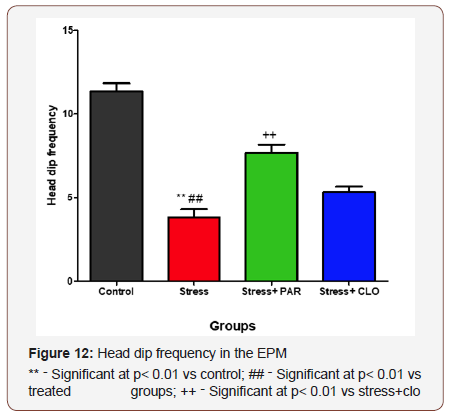
When comparing the stressed-treated groups, stressed+paroxatine group showed a significant increase (p<0.01) in head dip frequency.
Discussion and Conclusion
Discussion
Anxiety is a neurobehaviour that is determined using both the open field apparatus and the elevated-plus maze, both of which were utilized in the course of this study. Behavior such as Grooming, Rearing, Head dip, Centre Square Entry (CSE) and Centre Square Duration (CSD) are all measures of anxiety [13,14]. An increase in Rearing, CSE, CSD and Head dip indicates decrease in the level of anxiety and vice-versa. An increase in Grooming frequency and Grooming duration both indicate an increase in the level of anxiety and vice-versa. There was a reported significant increase in anxiolytic effects of the stressed mice when compared with control mice, while the stress-treated with paroxatine and clomipramine mice both showed a decrease in anxiolytic effects compared to the stressed mice [14,15]. It was also further observed that, the stresstreated mice with paroxatine reduces anxiety significantly compared to the stress-treated mice with clomipramine. This reduction may be as a result of lack of anticholinergic effect of paroxatine which is one of the major advances of the SSRIs antidepressants. TCAs, apart from acting as strong inhibitors in the reuptake of both norepinephine and serotonin, also blocks histaminic, cholinergic, and alpha 1-adrenergic receptor sites, and this lack of selectivity is what accounts for the unwanted side effects such as weight gain, dry mouth, constipation, drowsiness, and dizziness. Unlike the TCAs, SSRIs do not cause anticholinergic, hypotensive or sedating reactions, and are not associated with impaired cognitive function [14-16]. It can therefore be suggested that, paroxatine enhances endogenous acetylcholine in the dorsal hippocampus thereby reducing anxiety through the actions on nicotinic and muscarinic1 receptors.
Conclusion
Both Paroxatine and Clomipramine significantly reduce anxiety in stress. But Paroxantine reduces it significantly than clomipramine when compared. This hyper effect could be due to the selectivity properties of paroxatine which do not affect other neurotransmitters like histamine and acetylcholine, thereby causing the blockage of histaminic and cholinergic receptor sites. It may therefore be necessary to recommend paroxatine as the first line of treatment for enhancing minimizing anxiolytic effects in stress.
In the memory, orientation, and praxis areas, the averages obtained were negative because the average was calculated by subtracting the result of epileptic patients from healthy patients. Since the established hypothesis was that healthy subjects should have a higher score than patients with epilepsy, surprisingly, when applying the statistical test, it was found in all the items analyzed that there was no statistically significant difference. When making the comparison in the areas of memory, language, orientation, praxis, attention and, calculation using the global epileptic patients and healthy subjects that were demographically equal by age and schooling, the results described in Table 2 were found (Table 2).
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Friedman HS (2007) Personality, disease, and self-healing. In: Friedman HS, Silver RC, editors. Foundations of Health Psychology. NY: Oxford University Press, USA, pp. 172-199.
- Salmans Sandra (1997) Depression: Questions You Have – Answers You Need. People’s Medical Society. ISBN 978-1-882606-14-6.
- Kumar TP, Antony S, Gireesh G, George N, Paulose CS (2010) Curcumin modulates dopaminergic receptor, CREB and phospholipase C gene expression in the cerebral cortex and cerebellum of streptozotocin induced diabetic rats. Journal of biomedical science 17(1): 43.
- WHO (2012) GENEVA - On World Mental Health Day (10 October), WHO Calls is for an end to the stigmatization of depression and other mental disorders and for better access to treatment for all people who need it.
- Salvatore P, Indic P, Murray G, Baldessarini RJ (2012) Biological rhythms and mood disorders. Dialogues in clinical neuroscience 14(4): 369.
- Linde K, Kriston L, Rücker G, Jamil S, Schumann I, et al. (2015) Efficacy and acceptability of pharmacological treatments for depressive disorders in primary care: systematic review and network meta-analysis. The Annals of Family Medicine 13(1): pp. 69-79.
- Amy HF, Shamsah BS, Daniel PJ, Rebecca H (2010) Early improvements in anxiety, depression, and anger/hostility symptoms and response to antidepressant treatment, Annals of Clinical Psychiatry 22(4): 13-21.
- Lepola U, Bergtholdt B, St Lambert J, Davy KL, Ruggiero L (2004) Controlled-release paroxetine in the treatment of patients with social anxiety disorder. J Clin Psychiatry 65: 222-229.
- Brunton PJ, Russell JA (2010) Prenatal Social Stress in the Rat Programmes Neuroendocrine and Behavioural Responses to Stress in the Adult Offspring: Sex‐Specific Effects. Journal of neuroendocrinology 22(4): 258-271.
- Guyton AC, Hall JE (2006) Textbook of Medical Physiology. 11th ed. Philadelphia, Penn: Elsevier/Saunders.
- Adrian C, Audrey M, Scott S Tiffany F, Bethany S (2012) A method for reliable voluntary oral administration of a fixed dosage (mg/kg) of chronic daily medication to rats. Laboratory Animals SAGE Journals 46(4): 318-324.
- Matsumoto R, Akama K, Rakwal R, Iwahashi H (2005) The stress response against denatured proteins in the deletion of cytosolic chaperones SSA1/2 is different from heat-shock response in Saccharomyces cerevisiae. BMC Genomics 6: 141
- Brown RE, Corey SC, Moore AK (1999) Differences in measures of exploration and fear in MHC – Congenic C57BL/6J and B6 – H – 2K mice. Behaviour Genetics 26: 263-271.
- McFadyen MP, Brown RE, Carrey N (2002) Subchronic methylphenidate administration has no effect on locomotion, emotional behavior, or water maze learning in prepubertal mice. Developmental psychobiology 41: 123-132.
- Podhorna J, Brown RE (2002) Stain differences in activity and emotionality do not account for differences in learning and memory performance between C57BL/6 and DBA/2 mice. Genes, Brain and Behavior 1: 96–110.
-
Mfem CC, Nyoro IK, Seriki SA. Comparative Role of Paroxatine and Clomipramine on Anxiety in Mice Recovering from Stress. Arch Neurol & Neurosci. 4(4): 2019. ANN.MS.ID.000594.
-
Anxiety; Stress; Paroxatine; Clomipramine
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






